
Global Gene Therapy for Spinal Muscular Atrophy Market Size, Trends & Analysis - Forecasts to 2029 By Therapy Type (AAV-based Gene Therapy and Lentiviral vector-based Gene Therapy), By Development Phase (Preclinical, Clinical (Phase I, II, III), and Commercialized), and By Region (North America, Asia Pacific, Central & South America, Europe, and Middle East and Africa), Competitive Landscape, Company Market Share Analysis, and End User Analysis
The global gene therapy for spinal muscular atrophy (SMA) market is analyzed to grow at a CAGR of 7.6% during the forecast period from 2024 to 2029. Gene therapy has emerged as a promising treatment approach for SMA, particularly with the development of drugs like onasemnogene abeparvovec (Zolgensma) and nusinersen (Spinraza).
The market is driven by advancements in gene therapy technology and increasing regulatory approvals. Continuous advancements in gene therapy technology, including viral vectors and gene-editing tools like CRISPR-Cas9, drive innovation in SMA treatment. These technological advancements enable more efficient delivery of therapeutic genes and potentially enhance gene therapy's effectiveness for SMA. The approval of gene therapy drugs by regulatory agencies such as the FDA has boosted confidence in gene therapy as a viable treatment option for SMA. Regulatory approvals not only validate the efficacy and safety of gene therapy but also facilitate market penetration and adoption, driving growth in the SMA gene therapy market. The chance to broaden the range of treatments beyond traditional methods such as Spinraza to incorporate gene therapy presents new opportunities for individuals with SMA. Gene therapy holds promise for delivering lasting effects with just one treatment, offering hope for better results and enhanced quality of life for SMA patients spanning various ages and severity levels of the disease.
One of the primary restraints in the SMA gene therapy market is the high cost associated with treatment. The high cost of treatment limits access and affordability, particularly in regions with limited healthcare resources, thereby restraining market growth and adoption.
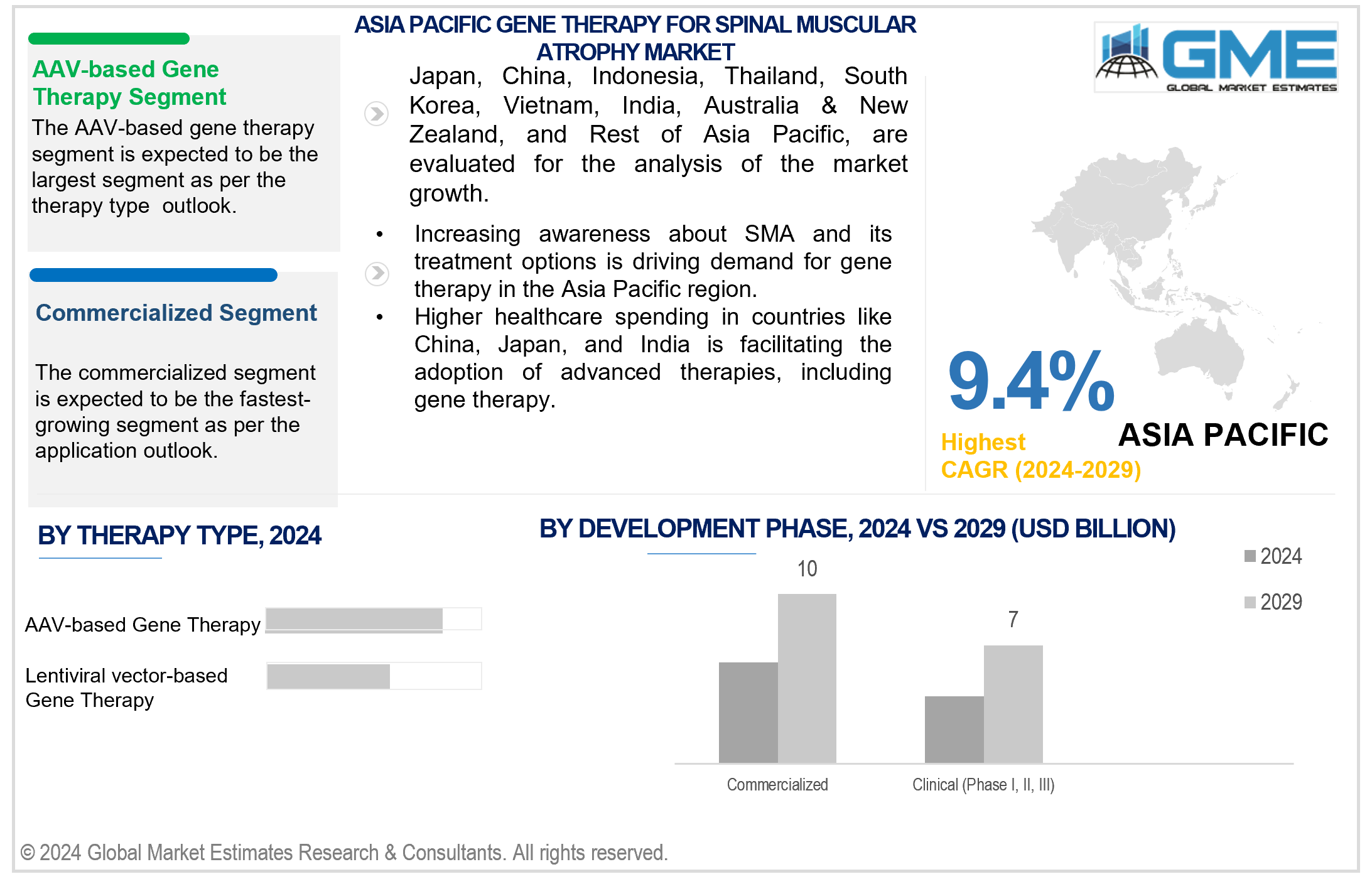
Based on therapy type, the market is segmented into AAV-based gene therapy and lentiviral vector-based gene therapy. The AAV-based gene therapy segment is expected to hold the largest share of the market during the forecast period. AAV-based gene therapy has become the preferred treatment for SMA due to its proven effectiveness and safety. This therapy utilizes adeno-associated viral vectors (AAV) to deliver functional copies of the defective SMN1 gene, addressing the root cause of the disease. Clinical trials have demonstrated its ability to significantly improve motor function and prolong survival in SMA patients, leading to widespread adoption by healthcare providers and patients seeking long-term therapeutic benefits with minimal risks.
The lentiviral vector-based gene therapy segment is projected to grow fastest during the forecast period. Lentiviral vector-based gene therapy is rapidly gaining traction as a treatment for SMA. Its growth is fueled by advancements in vector design and delivery methods, enhancing its ability to effectively deliver therapeutic genes. Additionally, promising results from preclinical and clinical trials have bolstered confidence in its potential to provide long-lasting benefits for SMA patients. These factors contribute to its status as the fastest-growing segment in the gene therapy market for SMA.
Based on development phase, the market is segmented into preclinical, clinical (Phase I, II, III), and commercialized. The commercial segment is expected to hold the largest share of the market during the forecast period. It includes gene therapy drugs that have obtained regulatory approval and are accessible for clinical use. These approved therapies have undergone rigorous testing to ensure their safety and efficacy, making them available to healthcare providers and patients for the treatment of SMA, thereby significantly impacting the management and outcomes of the disease.
The clinical (Phase I, II, III) segment is projected to grow fastest during the forecast period. This surge can be attributed to ongoing research and development efforts to advance gene therapy candidates through various stages of clinical trials. Phase III trials, in particular, play a crucial role in demonstrating the efficacy and safety of potential treatments, driving the segment's rapid expansion.
North America is analyzed to be the largest region in the global gene therapy for spinal muscular atrophy market during the forecast period. This is due to robust healthcare infrastructure, high R&D investments, a supportive regulatory environment, and early adoption of innovative therapies.
Asia Pacific is analyzed to be the fastest-growing region in the gene therapy for spinal muscular atrophy market during the forecast period. Increasing awareness about SMA and its treatment options drives demand for gene therapy in the Asia Pacific region. Higher healthcare spending in countries like China, Japan, and India facilitates the adoption of advanced therapies, including gene therapy. The Asia Pacific region has a significant population base, contributing to a growing pool of SMA patients seeking advanced treatment options like gene therapy. Growing investments by pharmaceutical companies and biotech firms in the Asia Pacific region are fueling the development and commercialization of gene therapy treatments for SMA.
Novartis AG, Biogen Inc., Roche Holding AG, Pfizer Inc., Regenxbio Inc., Solid Biosciences Inc., Sarepta Therapeutics Inc., and Genethon, among others, are some of the key players operating in the global market.
Please note: This is not an exhaustive list of companies profiled in the report.
In June 2023, Biogen Inc. announced new SPINRAZA (nusinersen) data aimed at answering critical questions for the spinal muscular atrophy (SMA) community. The data were presented at the SMA Research & Clinical Care Meeting hosted by Cure SMA in Orlando.
In March 2024, REGENXBIO Inc. reported additional interim safety and efficacy data in the Phase I/II AFFINITY DUCHENNE trial of RGX-202 in patients with Duchenne muscular dystrophy (Duchenne) ages 4 to11 years old, including RGX-202 microdystrophin expression from dose level 2 and video of trial clinic assessments demonstrating initial evidence of strength and functional improvement.
1 STRATEGIC INSIGHTS ON NEW REVENUE POCKETS
1.1 Strategic Opportunity & Attractiveness Analysis
1.1.1 Hot Revenue Pockets
1.1.2 Market Attractiveness Score
1.1.3 Revenue Impacting Opportunity
1.1.4 High Growing Region/Country
1.1.5 Competitor Analysis
1.1.6 Consumer Analysis
1.2 Global Market Estimates' View
1.3 Strategic Insights across Business Functions
1.3.1 For Chief Executive Officers
1.3.2 For Chief Marketing Officers
1.3.3 For Chief Strategy Officers
1.4 Evaluate the Potential of your Existing Business Lines vs. New Lines to Enter Into
2 TECHNOLOGICAL TRENDS
2.1 Technological Adoption Rate
2.2 Current Trend Impact Analysis
2.3 Future Trend Impact Analysis
3 GLOBAL MARKET OUTLOOK
3.1 Market Pyramid Analysis
3.1.1 Introduction
3.1.2 Adjacent Market Opportunities
3.1.3 Ancillary Market Opportunities
3.2 Demand Side Analysis
3.2.1 Market Drivers: Impact Analysis
3.2.2 Market Restraints: Impact Analysis
3.2.3 Market Opportunities: Impact Analysis
3.2.4 Market Challenges: Impact Analysis
3.3 Supply Side Analysis
3.3.1 Porter’s Five Forces Analysis
3.3.1.1 Threat of New Entrants
3.3.1.2 Threat of New Substitutes
3.3.1.3 Bargaining Power of Suppliers
3.3.1.4 Bargaining Power of Buyers
3.3.1.5 Intensity of Competitive Rivalry
3.3.2 SWOT Analysis; By Factor (Political & Legal, Economic, and Technological)
3.3.2.1 Political Landscape
3.3.2.2 Economic Landscape
3.3.2.3 Social Landscape
3.3.2.4 Technology Landscape
3.3.3 Value Chain Analysis
3.3.4 Trend Analysis
3.3.5 Gap Analysis
3.3.6 Cost Analysis
4 GLOBAL GENE THERAPY FOR SPINAL MUSCULAR ATROPHY MARKET, BY THERAPY TYPE
4.1 Introduction
4.2 Gene Therapy for Spinal Muscular Atrophy Market: Therapy Type Scope Key Takeaways
4.3 Revenue Growth Analysis, 2023 & 2029
4.4 AAV-based Gene Therapy
4.4.1 AAV-based Gene Therapy Market Estimates and Forecast, 2021-2029 (USD Million)
4.5 Lentiviral vector-based Gene Therapy
4.5.1 Lentiviral vector-based Gene Therapy Market Estimates and Forecast, 2021-2029 (USD Million)
5 GLOBAL GENE THERAPY FOR SPINAL MUSCULAR ATROPHY MARKET, BY DEVELOPMENT PHASE
5.1 Introduction
5.2 Gene Therapy for Spinal Muscular Atrophy Market: Development Phase Scope Key Takeaways
5.3 Revenue Growth Analysis, 2023 & 2029
5.4 Preclinical
5.4.1 Preclinical Market Estimates and Forecast, 2021-2029 (USD Million)
5.5 Clinical (Phase I, II, III)
5.5.1 Clinical (Phase I, II, III) Market Estimates and Forecast, 2021-2029 (USD Million)
5.6 Commercialized
5.6.1 Commercialized Market Estimates and Forecast, 2021-2029 (USD Million)
6 GLOBAL GENE THERAPY FOR SPINAL MUSCULAR ATROPHY MARKET, BY REGION
6.1 Introduction
6.2 North America Gene Therapy for Spinal Muscular Atrophy Market Estimates and Forecast, 2021-2029 (USD Million)
6.2.1 By Therapy Type
6.2.2 By Development Phase
6.2.3 By Country
6.2.3.1 U.S. Gene Therapy for Spinal Muscular Atrophy Market Estimates and Forecast, 2021-2029 (USD Million)
6.2.3.1.1 By Therapy Type
6.2.3.1.2 By Development Phase
6.2.3.2 Canada Gene Therapy for Spinal Muscular Atrophy Market Estimates and Forecast, 2021-2029 (USD Million)
6.2.3.2.1 By Therapy Type
6.2.3.2.2 By Development Phase
6.2.3.3 Mexico Gene Therapy for Spinal Muscular Atrophy Market Estimates and Forecast, 2021-2029 (USD Million)
6.2.3.3.1 By Therapy Type
6.2.3.3.2 By Development Phase
6.3 Europe Gene Therapy for Spinal Muscular Atrophy Market Estimates and Forecast, 2021-2029 (USD Million)
6.3.1 By Therapy Type
6.3.2 By Development Phase
6.3.3 By Country
6.3.3.1 Germany Gene Therapy for Spinal Muscular Atrophy Market Estimates and Forecast, 2021-2029 (USD Million)
6.3.3.1.1 By Therapy Type
6.3.3.1.2 By Development Phase
6.3.3.2 U.K. Gene Therapy for Spinal Muscular Atrophy Market Estimates and Forecast, 2021-2029 (USD Million)
6.3.3.2.1 By Therapy Type
6.3.3.2.2 By Development Phase
6.3.3.3 France Gene Therapy for Spinal Muscular Atrophy Market Estimates and Forecast, 2021-2029 (USD Million)
6.3.3.3.1 By Therapy Type
6.3.3.3.2 By Development Phase
6.3.3.4 Italy Gene Therapy for Spinal Muscular Atrophy Market Estimates and Forecast, 2021-2029 (USD Million)
6.3.3.4.1 By Therapy Type
6.3.3.4.2 By Development Phase
6.3.3.5 Spain Gene Therapy for Spinal Muscular Atrophy Market Estimates and Forecast, 2021-2029 (USD Million)
6.3.3.5.1 By Therapy Type
6.3.3.5.2 By Development Phase
6.3.3.6 Netherlands Gene Therapy for Spinal Muscular Atrophy Market Estimates and Forecast, 2021-2029 (USD Million)
6.3.3.6.1 By Therapy Type
6.3.3.6.2 By Development Phase
6.3.3.7 Rest of Europe Gene Therapy for Spinal Muscular Atrophy Market Estimates and Forecast, 2021-2029 (USD Million)
6.3.3.6.1 By Therapy Type
6.3.3.6.2 By Development Phase
6.4 Asia Pacific Gene Therapy for Spinal Muscular Atrophy Market Estimates and Forecast, 2021-2029 (USD Million)
6.4.1 By Therapy Type
6.4.2 By Development Phase
6.4.3 By Country
6.4.3.1 China Gene Therapy for Spinal Muscular Atrophy Market Estimates and Forecast, 2021-2029 (USD Million)
6.4.3.1.1 By Therapy Type
6.4.3.1.2 By Development Phase
6.4.3.2 Japan Gene Therapy for Spinal Muscular Atrophy Market Estimates and Forecast, 2021-2029 (USD Million)
6.4.3.2.1 By Therapy Type
6.4.3.2.2 By Development Phase
6.4.3.3 India Gene Therapy for Spinal Muscular Atrophy Market Estimates and Forecast, 2021-2029 (USD Million)
6.4.3.3.1 By Therapy Type
6.4.3.3.2 By Development Phase
6.4.3.4 South Korea Gene Therapy for Spinal Muscular Atrophy Market Estimates and Forecast, 2021-2029 (USD Million)
6.4.3.4.1 By Therapy Type
6.4.3.4.2 By Development Phase
6.4.3.5 Singapore Gene Therapy for Spinal Muscular Atrophy Market Estimates and Forecast, 2021-2029 (USD Million)
6.4.3.5.1 By Therapy Type
6.4.3.5.2 By Development Phase
6.4.3.6 Malaysia Gene Therapy for Spinal Muscular Atrophy Market Estimates and Forecast, 2021-2029 (USD Million)
6.4.3.6.1 By Therapy Type
6.4.3.6.2 By Development Phase
6.4.3.7 Thailand Gene Therapy for Spinal Muscular Atrophy Market Estimates and Forecast, 2021-2029 (USD Million)
6.4.3.6.1 By Therapy Type
6.4.3.6.2 By Development Phase
6.4.3.8 Indonesia Gene Therapy for Spinal Muscular Atrophy Market Estimates and Forecast, 2021-2029 (USD Million)
6.4.3.7.1 By Therapy Type
6.4.3.7.2 By Development Phase
6.4.3.9 Vietnam Gene Therapy for Spinal Muscular Atrophy Market Estimates and Forecast, 2021-2029 (USD Million)
6.4.3.8.1 By Therapy Type
6.4.3.8.2 By Development Phase
6.4.3.10 Taiwan Gene Therapy for Spinal Muscular Atrophy Market Estimates and Forecast, 2021-2029 (USD Million)
6.4.3.10.1 By Therapy Type
6.4.3.10.2 By Development Phase
6.4.3.11 Rest of Asia Pacific Gene Therapy for Spinal Muscular Atrophy Market Estimates and Forecast, 2021-2029 (USD Million)
6.4.3.11.1 By Therapy Type
6.4.3.11.2 By Development Phase
6.5 Middle East and Africa Gene Therapy for Spinal Muscular Atrophy Market Estimates and Forecast, 2021-2029 (USD Million)
6.5.1 By Therapy Type
6.5.2 By Development Phase
6.5.3 By Country
6.5.3.1 Saudi Arabia Gene Therapy for Spinal Muscular Atrophy Market Estimates and Forecast, 2021-2029 (USD Million)
6.5.3.1.1 By Therapy Type
6.5.3.1.2 By Development Phase
6.5.3.2 U.A.E. Gene Therapy for Spinal Muscular Atrophy Market Estimates and Forecast, 2021-2029 (USD Million)
6.5.3.2.1 By Therapy Type
6.5.3.2.2 By Development Phase
6.5.3.3 Israel Gene Therapy for Spinal Muscular Atrophy Market Estimates and Forecast, 2021-2029 (USD Million)
6.5.3.3.1 By Therapy Type
6.5.3.3.2 By Development Phase
6.5.3.4 South Africa Gene Therapy for Spinal Muscular Atrophy Market Estimates and Forecast, 2021-2029 (USD Million)
6.5.3.4.1 By Therapy Type
6.5.3.4.2 By Development Phase
6.5.3.5 Rest of Middle East and Africa Gene Therapy for Spinal Muscular Atrophy Market Estimates and Forecast, 2021-2029 (USD Million)
6.5.3.5.1 By Therapy Type
6.5.3.5.2 By Development Phase
6.6 Central & South America Gene Therapy for Spinal Muscular Atrophy Market Estimates and Forecast, 2021-2029 (USD Million)
6.6.1 By Therapy Type
6.6.2 By Development Phase
6.6.3 By Country
6.6.3.1 Brazil Gene Therapy for Spinal Muscular Atrophy Market Estimates and Forecast, 2021-2029 (USD Million)
6.6.3.1.1 By Therapy Type
6.6.3.1.2 By Development Phase
6.6.3.2 Argentina Gene Therapy for Spinal Muscular Atrophy Market Estimates and Forecast, 2021-2029 (USD Million)
6.6.3.2.1 By Therapy Type
6.6.3.2.2 By Development Phase
6.6.3.3 Chile Gene Therapy for Spinal Muscular Atrophy Market Estimates and Forecast, 2021-2029 (USD Million)
6.6.3.3.1 By Therapy Type
6.6.3.3.2 By Development Phase
6.6.3.3 Rest of Central & South America Gene Therapy for Spinal Muscular Atrophy Market Estimates and Forecast, 2021-2029 (USD Million)
6.6.3.3.1 By Therapy Type
6.6.3.3.2 By Development Phase
7 COMPETITIVE LANDCAPE
7.1 Company Market Share Analysis
7.2 Four Quadrant Positioning Matrix
7.2.1 Market Leaders
7.2.2 Market Visionaries
7.2.3 Market Challengers
7.2.4 Niche Market Players
7.3 Vendor Landscape
7.3.1 North America
7.3.2 Europe
7.3.3 Asia Pacific
7.3.4 Rest of the World
7.4 Company Profiles
7.4.1 Novartis AG
7.4.1.1 Business Description & Financial Analysis
7.4.1.2 SWOT Analysis
7.4.1.3 Products & Services Offered
7.4.1.4 Strategic Alliances between Business Partners
7.4.2 Biogen Inc.
7.4.2.1 Business Description & Financial Analysis
7.4.2.2 SWOT Analysis
7.4.2.3 Products & Services Offered
7.4.2.4 Strategic Alliances between Business Partners
7.4.3 Roche Holding AG
7.4.3.1 Business Description & Financial Analysis
7.4.3.2 SWOT Analysis
7.4.3.3 Products & Services Offered
7.4.3.4 Strategic Alliances between Business Partners
7.4.4 Pfizer Inc.
7.4.4.1 Business Description & Financial Analysis
7.4.4.2 SWOT Analysis
7.4.4.3 Products & Services Offered
7.4.4.4 Strategic Alliances between Business Partners
7.4.5 Regenxbio Inc.
7.4.5.1 Business Description & Financial Analysis
7.4.5.2 SWOT Analysis
7.4.5.3 Products & Services Offered
7.4.5.4 Strategic Alliances between Business Partners
7.4.6 SOLID BIOSCIENCES INC.
7.4.6.1 Business Description & Financial Analysis
7.4.6.2 SWOT Analysis
7.4.6.3 Products & Services Offered
7.4.6.4 Strategic Alliances between Business Partners
7.4.7 Sarepta Therapeutics Inc.
7.4.7.1 Business Description & Financial Analysis
7.4.7.2 SWOT Analysis
7.4.7.3 Products & Services Offered
7.4.7.4 Strategic Alliances between Business Partners
7.4.8 Genethon
7.4.8.1 Business Description & Financial Analysis
7.4.8.2 SWOT Analysis
7.4.8.3 Products & Services Offered
7.4.8.4 Strategic Alliances between Business Partners
7.4.9 Other Companies
7.4.9.1 Business Description & Financial Analysis
7.4.9.2 SWOT Analysis
7.4.9.3 Products & Services Offered
7.4.9.4 Strategic Alliances between Business Partners
8 RESEARCH METHODOLOGY
8.1 Market Introduction
8.1.1 Market Definition
8.1.2 Market Scope & Segmentation
8.2 Information Procurement
8.2.1 Secondary Research
8.2.1.1 Purchased Databases
8.2.1.2 GMEs Internal Data Repository
8.2.1.3 Secondary Resources & Third Party Perspectives
8.2.1.4 Company Information Sources
8.2.2 Primary Research
8.2.2.1 Various Types of Respondents for Primary Interviews
8.2.2.2 Number of Interviews Conducted throughout the Research Process
8.2.2.3 Primary Stakeholders
8.2.2.4 Discussion Guide for Primary Participants
8.2.3 Expert Panels
8.2.3.1 Expert Panels Across 30+ Industry
8.2.4 Paid Local Experts
8.2.4.1 Paid Local Experts Across 30+ Industry Across each Region
8.3 Market Estimation
8.3.1 Top-Down Approach
8.3.1.1 Macro-Economic Indicators Considered
8.3.1.2 Micro-Economic Indicators Considered
8.3.2 Bottom Up Approach
8.3.2.1 Company Share Analysis Approach
8.3.2.2 Estimation of Potential Product Sales
8.4 Data Triangulation
8.4.1 Data Collection
8.4.2 Time Series, Cross Sectional & Panel Data Analysis
8.4.3 Cluster Analysis
8.5 Analysis and Output
8.5.1 Inhouse AI Based Real Time Analytics Tool
8.5.2 Output From Desk & Primary Research
8.6 Research Assumptions & Limitations
8.6.1 Research Assumptions
8.6.2 Research Limitations
LIST OF TABLES
1 Global Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type, 2021-2029 (USD Million)
2 AAV-based Gene Therapy Market, By Region, 2021-2029 (USD Million)
3 Lentiviral vector-based Gene Therapy Market, By Region, 2021-2029 (USD Million)
4 Global Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase, 2021-2029 (USD Million)
5 Preclinical Market, By Region, 2021-2029 (USD Million)
6 Clinical (Phase I, II, III) Market, By Region, 2021-2029 (USD Million)
7 Commercialized Market, By Region, 2021-2029 (USD Million)
8 Regional Analysis, 2021-2029 (USD Million)
9 North America Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type, 2021-2029 (USD Million)
10 North America Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase, 2021-2029 (USD Million)
11 North America Gene Therapy for Spinal Muscular Atrophy Market, By Country, 2021-2029 (USD Million)
12 U.S. Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type, 2021-2029 (USD Million)
13 U.S. Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase, 2021-2029 (USD Million)
14 Canada Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type, 2021-2029 (USD Million)
15 Canada Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase, 2021-2029 (USD Million)
16 Mexico Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type, 2021-2029 (USD Million)
17 Mexico Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase, 2021-2029 (USD Million)
18 Europe Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type, 2021-2029 (USD Million)
19 Europe Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase, 2021-2029 (USD Million)
20 Europe Gene Therapy for Spinal Muscular Atrophy Market, By Country, 2021-2029 (USD Million)
21 Germany Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type, 2021-2029 (USD Million)
22 Germany Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase, 2021-2029 (USD Million)
23 U.K. Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type, 2021-2029 (USD Million)
24 U.K. Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase, 2021-2029 (USD Million)
25 France Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type, 2021-2029 (USD Million)
26 France Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase, 2021-2029 (USD Million)
27 Italy Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type, 2021-2029 (USD Million)
28 Italy Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase, 2021-2029 (USD Million)
29 Spain Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type, 2021-2029 (USD Million)
30 Spain Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase, 2021-2029 (USD Million)
31 Netherlands Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type, 2021-2029 (USD Million)
32 Netherlands Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase, 2021-2029 (USD Million)
33 Rest Of Europe Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type, 2021-2029 (USD Million)
34 Rest Of Europe Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase, 2021-2029 (USD Million)
35 Asia Pacific Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type, 2021-2029 (USD Million)
36 Asia Pacific Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase, 2021-2029 (USD Million)
37 Asia Pacific Gene Therapy for Spinal Muscular Atrophy Market, By Country, 2021-2029 (USD Million)
38 China Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type, 2021-2029 (USD Million)
39 China Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase, 2021-2029 (USD Million)
40 Japan Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type, 2021-2029 (USD Million)
41 Japan Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase, 2021-2029 (USD Million)
42 India Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type, 2021-2029 (USD Million)
43 India Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase, 2021-2029 (USD Million)
44 South Korea Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type, 2021-2029 (USD Million)
45 South Korea Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase, 2021-2029 (USD Million)
46 Singapore Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type, 2021-2029 (USD Million)
47 Singapore Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase, 2021-2029 (USD Million)
48 Thailand Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type, 2021-2029 (USD Million)
49 Thailand Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase, 2021-2029 (USD Million)
50 Malaysia Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type, 2021-2029 (USD Million)
51 Malaysia Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase, 2021-2029 (USD Million)
52 Indonesia Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type, 2021-2029 (USD Million)
53 Indonesia Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase, 2021-2029 (USD Million)
54 Vietnam Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type, 2021-2029 (USD Million)
55 Vietnam Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase, 2021-2029 (USD Million)
56 Taiwan Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type, 2021-2029 (USD Million)
57 Taiwan Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase, 2021-2029 (USD Million)
58 Rest of APAC Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type, 2021-2029 (USD Million)
59 Rest of APAC Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase, 2021-2029 (USD Million)
60 Middle East and Africa Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type, 2021-2029 (USD Million)
61 Middle East and Africa Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase, 2021-2029 (USD Million)
62 Middle East and Africa Gene Therapy for Spinal Muscular Atrophy Market, By country, 2021-2029 (USD Million)
63 Saudi Arabia Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type, 2021-2029 (USD Million)
64 Saudi Arabia Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase, 2021-2029 (USD Million)
65 UAE Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type, 2021-2029 (USD Million)
66 UAE Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase, 2021-2029 (USD Million)
67 Israel Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type, 2021-2029 (USD Million)
68 Israel Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase, 2021-2029 (USD Million)
69 South Africa Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type, 2021-2029 (USD Million)
70 South Africa Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase, 2021-2029 (USD Million)
71 Rest Of Middle East and Africa Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type, 2021-2029 (USD Million)
72 Rest Of Middle East and Africa Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase, 2021-2029 (USD Million)
73 Central & South America Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type, 2021-2029 (USD Million)
74 Central & South America Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase, 2021-2029 (USD Million)
75 Central & South America Gene Therapy for Spinal Muscular Atrophy Market, By Country, 2021-2029 (USD Million)
76 Brazil Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type, 2021-2029 (USD Million)
77 Brazil Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase, 2021-2029 (USD Million)
78 Chile Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type, 2021-2029 (USD Million)
79 Chile Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase, 2021-2029 (USD Million)
80 Argentina Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type, 2021-2029 (USD Million)
81 Argentina Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase, 2021-2029 (USD Million)
82 Rest Of Central & South America Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type, 2021-2029 (USD Million)
83 Rest Of Central & South America Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase, 2021-2029 (USD Million)
84 Novartis AG: Products & Services Offering
85 Biogen Inc.: Products & Services Offering
86 Roche Holding AG: Products & Services Offering
87 Pfizer Inc.: Products & Services Offering
88 Regenxbio Inc.: Products & Services Offering
89 SOLID BIOSCIENCES INC.: Products & Services Offering
90 Sarepta Therapeutics Inc. : Products & Services Offering
91 Genethon: Products & Services Offering
92 Other Companies: Products & Services Offering
LIST OF FIGURES
1 Global Gene Therapy for Spinal Muscular Atrophy Market Overview
2 Global Gene Therapy for Spinal Muscular Atrophy Market Value From 2021-2029 (USD Million)
3 Global Gene Therapy for Spinal Muscular Atrophy Market Share, By Therapy Type (2023)
4 Global Gene Therapy for Spinal Muscular Atrophy Market Share, By Development Phase (2023)
5 Global Gene Therapy for Spinal Muscular Atrophy Market, By Region (Asia Pacific Market)
6 Technological Trends In Global Gene Therapy for Spinal Muscular Atrophy Market
7 Four Quadrant Competitor Positioning Matrix
8 Impact Of Macro & Micro Indicators On The Market
9 Impact Of Key Drivers On The Global Gene Therapy for Spinal Muscular Atrophy Market
10 Impact Of Challenges On The Global Gene Therapy for Spinal Muscular Atrophy Market
11 Porter’s Five Forces Analysis
12 Global Gene Therapy for Spinal Muscular Atrophy Market: By Therapy Type Scope Key Takeaways
13 Global Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type Segment: Revenue Growth Analysis
14 AAV-based Gene Therapy Market, By Region, 2021-2029 (USD Million)
15 Lentiviral vector-based Gene Therapy Market, By Region, 2021-2029 (USD Million)
16 Global Gene Therapy for Spinal Muscular Atrophy Market: By Development Phase Scope Key Takeaways
17 Global Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase Segment: Revenue Growth Analysis
18 Preclinical Market, By Region, 2021-2029 (USD Million)
19 Clinical (Phase I, II, III) Market, By Region, 2021-2029 (USD Million)
20 Commercialized Market, By Region, 2021-2029 (USD Million)
21 Regional Segment: Revenue Growth Analysis
22 Global Gene Therapy for Spinal Muscular Atrophy Market: Regional Analysis
23 North America Gene Therapy for Spinal Muscular Atrophy Market Overview
24 North America Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type
25 North America Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase
26 North America Gene Therapy for Spinal Muscular Atrophy Market, By Country
27 U.S. Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type
28 U.S. Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase
29 Canada Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type
30 Canada Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase
31 Mexico Gene Therapy for Spinal Muscular Atrophy Market, By Therapy Type
32 Mexico Gene Therapy for Spinal Muscular Atrophy Market, By Development Phase
33 Four Quadrant Positioning Matrix
34 Company Market Share Analysis
35 Novartis AG: Company Snapshot
36 Novartis AG: SWOT Analysis
37 Novartis AG: Geographic Presence
38 Biogen Inc.: Company Snapshot
39 Biogen Inc.: SWOT Analysis
40 Biogen Inc.: Geographic Presence
41 Roche Holding AG: Company Snapshot
42 Roche Holding AG: SWOT Analysis
43 Roche Holding AG: Geographic Presence
44 Pfizer Inc.: Company Snapshot
45 Pfizer Inc.: Swot Analysis
46 Pfizer Inc.: Geographic Presence
47 Regenxbio Inc.: Company Snapshot
48 Regenxbio Inc.: SWOT Analysis
49 Regenxbio Inc.: Geographic Presence
50 SOLID BIOSCIENCES INC.: Company Snapshot
51 SOLID BIOSCIENCES INC.: SWOT Analysis
52 SOLID BIOSCIENCES INC.: Geographic Presence
53 Sarepta Therapeutics Inc. : Company Snapshot
54 Sarepta Therapeutics Inc. : SWOT Analysis
55 Sarepta Therapeutics Inc. : Geographic Presence
56 Genethon: Company Snapshot
57 Genethon: SWOT Analysis
58 Genethon: Geographic Presence
59 Other Companies: Company Snapshot
60 Other Companies: SWOT Analysis
61 Other Companies: Geographic Presence
The Global Gene Therapy for Spinal Muscular Atrophy Market has been studied from the year 2019 till 2029. However, the CAGR provided in the report is from the year 2024 to 2029. The research methodology involved three stages: Desk research, Primary research, and Analysis & Output from the entire research process.

The desk research involved a robust background study which meant referring to paid and unpaid databases to understand the market dynamics; mapping contracts from press releases; identifying the key players in the market, studying their product portfolio, competition level, annual reports/SEC filings & investor presentations; and learning the demand and supply-side analysis for the Gene Therapy for Spinal Muscular Atrophy Market.

The primary research activity included telephonic conversations with more than 50 tier 1 industry consultants, distributors, and end-use product manufacturers.

Finally, based on the above thorough research process, an in-depth analysis was carried out considering the following aspects: market attractiveness, current & future market trends, market share analysis, SWOT analysis of the company and customer analytics.

Frequently Asked Questions
Tailor made solutions just for you
80% of our clients seek made-to-order reports. How do you want us to tailor yours?
OUR CLIENTS