
Global Corona Virus Vaccine Market Size, Trends & Analysis - Forecasts to 2026 By Vaccine Type (Inactivated Virus, Protein Subunit, Non-replicating Viral Vector, RNA Vaccine and Others [DNA Vaccine & Repurposed Vaccines]), By Technology (Vector-Based, Nucleic Acid-Based, Protein-Based and Whole Virus), By Patient Type (Adult & Geriatric Patients and Pediatric Patients), By Product Type (Multivalent Vaccine and Monovalent Vaccine), By Clinical Trial Phase (Phase 1, Phase 2, Phase 3 and Market Launched), By End-Users (Government Vaccination Centers, Hospitals & Clinics, E-Pharmacy Channel, Research & Development Organizations and Others), By Region (North America, Europe, Asia-Pacific, CSA, MEA), Competitive Landscape Company Market Share Analysis, and Competitor Analysis
Reported first in December 2019, in China’s Wuhan, coronavirus disease or famously called COVID-19 has become one of the most deadly virus-based diseases across the globe. As of March 2020, this disease had been declared a pandemic by World Health Organization (WHO). The virus/ SARS-CoV-2 heavily affects the lungs and the respiratory tract cells. As of 2021, with total cases of 16.3 crores across the globe and 33.8 lakhs deaths, this virus has attracted many pharmaceutical firms to launch vaccines, tests, drugs related to the treatment procedure of the disease. Also, as per the data provided by Coronavirus Disease (COVID-19) Weekly Epidemiological, around 57.8 million active cases and 1.3 million deaths due to this virus have been reported till 2020.
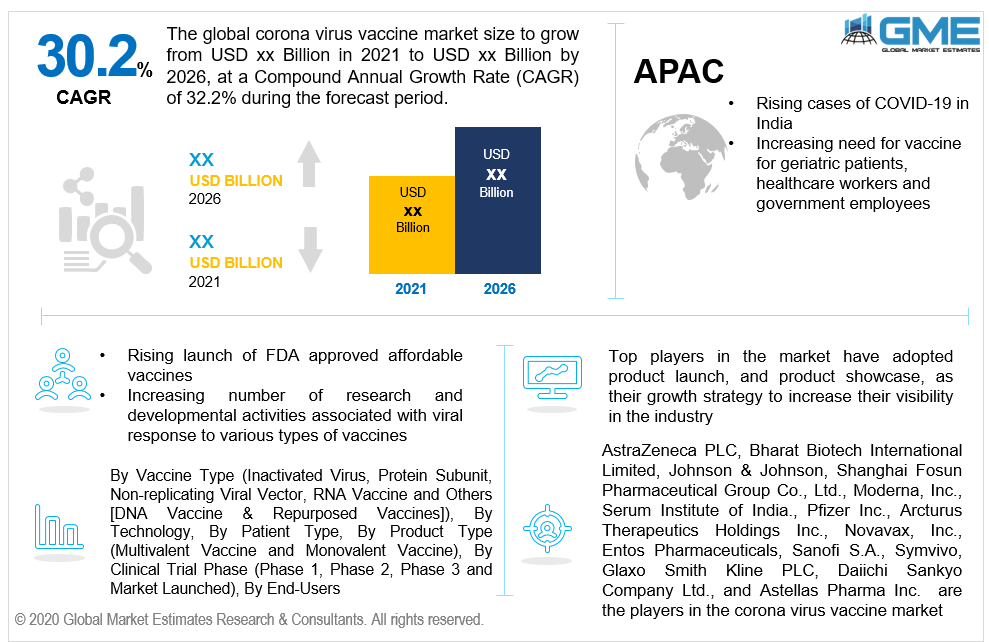
Because this virus is extremely contagious and can be easily transferred from one infected person to another healthy person, the market for its drug or vaccine ought to be on its toes and grow rapidly. Also, with the uncontrollable rising cases of COVID-19 especially in heavily populated countries, the industry is bound to get aggressively involved in offering top-notch vaccines (for preventive measures) or drugs/ medicines to treat and control the aftereffect of the viral attack. Hence, the rising COVID-19 cases and their complications have enunciated the need for COVID-19 vaccines, to control the spread and combat the pandemic situation. For instance, according to the World Health Organization (WHO), as of 12 November 2020, around 48 vaccines of the top pharmaceutical firms are currently undergoing a clinical evaluation process, and amongst which around 10 vaccine candidates are in Phase 3 clinical trials and 2-3 vaccines have been in use across the globe. Increasing government funds and financial support across the globe for the research and developmental activities related to developing a vaccine is another major factor for the coronavirus vaccine to grow rapidly. Government interest in the logistics of the vaccine will also support many manufacturers in launching their products successfully. However, due to strict regulatory norms imposed by the FDA for the approval of vaccines which are phase 3 trials will help the market growth restrict to some extent. As the pandemic has created a long time effect on the human population, the vaccine market is ought to be growing and be constant in terms of market revenue during the forecast period.
Based on the type of vaccines offered, the market can be classified as an inactivated virus, protein subunit, non-replicating viral vector, RNA vaccine, and others [DNA vaccine & repurposed vaccines]. Inactivated virus type vaccine will foresee a higher growth rate as compared to other types owing to the high demand of this type. Also, most of the manufacturers such as Sinovac, Sinopharm, Bharat Biotech, and others have taken a keen interest in developing vaccines of this type as the demand for vaccination has increased in developing nations. These companies are making efforts to meet the increasing demand for vaccines by opting for a low-cost and highly effective manufacturing process for the inactivated virus. However, the RNA vaccine segment will be growing the fastest owing to high economical comfort related to its manufacturing process and owing to its non-infectious source.
Based on technology, this market can be segmented into vector-based, nucleic acid-based, protein-based and whole virus. The vector-based vaccine will attain the highest market position in terms of revenue growth from 2021 to 2026, as this technology is considered to be the safest and advanced technology and yields the product more efficiently.
Adult & geriatric patients and pediatric patients are the categories of the patient type segment in the market. The adult & geriatric patients have been vaccinated the most during the last year and hence this segment holds the largest share in the coronavirus vaccine market. However, pediatric patient (vaccine given to children and newborn) segment will foresee the highest growth rate during the forecast period.
The corona virus vaccine market by product type can be seen to be segmented into the multivalent vaccine and monovalent vaccine. The monovalent vaccine being the most preferred vaccine type will be having the highest share in the market while the multivalent vaccine type will be growing with the highest CAGR value from 2021 to 2026.
As per the revenue generation is concerned, the market launched segment out of phases 1,2 and 3 clinical trial segment will be the largest segment from 2021 to 2026. However, as of 12 November 2020, around 48 vaccines of the top pharmaceutical firms are currently undergoing a clinical evaluation process, and among which around 10 vaccine candidates are in phase 3 clinical trials.
The end-user segment is divided into government vaccination centers, hospitals & clinics, e-pharmacy channels, research & development organizations, and others. The government vaccination center will be the largest end-user in terms of both volume and value in the market. This is so because the government has been working upon vaccinating most individuals to control the pandemic outbreak and avoid both economic and public loss. However, the e-commerce/ pharmacy channel will grow with the highest growth rate from 2021 to 2026, owing to increasing collaborative efforts of the healthcare industry and the online platforms for better reach and efficient productivity.
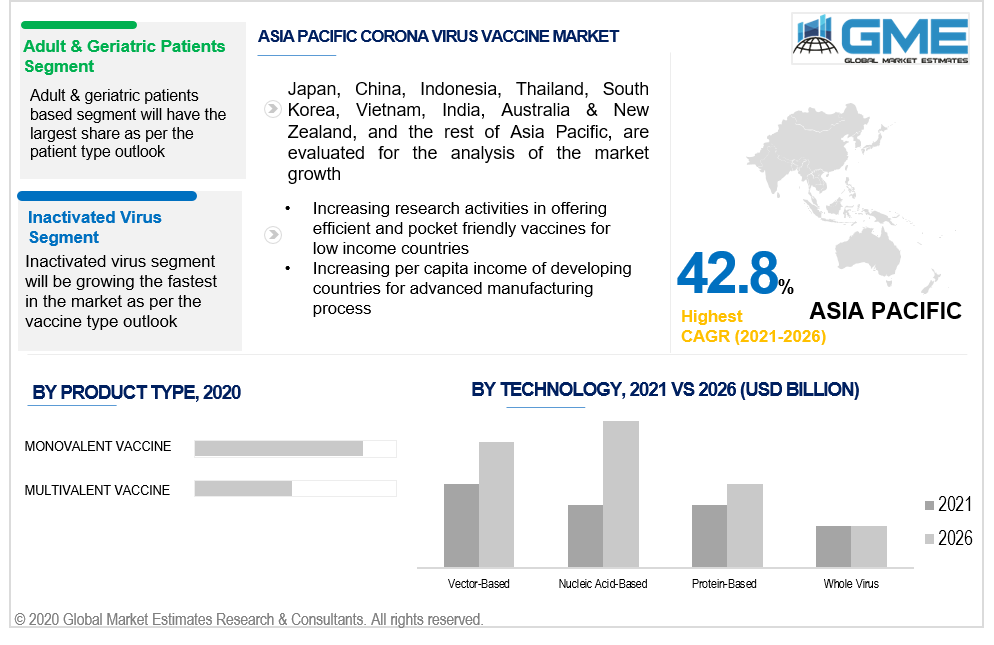
As per the geographical analysis, the corona virus vaccine market can be classified into North America (the United States, Canada, and Mexico), Asia Pacific (India, China, Japan, Malaysia, Singapore, and Rest of Asia Pacific), Europe (Germany, United Kingdom, Italy, France, Spain, Netherlands, and Rest of Europe), Middle East & Africa (Saudi Arabia, United Arab Emirates, and Rest of the Middle East & Africa) and Central & South America (Brazil, Argentina, and Rest of Central and South America). Based on the number of vaccinations done, the North American market will be the largest both in terms of value and volume during the forecast period. However, with the uncontrollable outbreak in the heavily populated Asian countries, the market here will be growing the fastest from 2021 to 2026.
AstraZeneca PLC, Bharat Biotech International Limited, Johnson & Johnson, Sinovac Biotech Ltd., BioNTech SE, Cadila Healthcare Limited, Shanghai Fosun Pharmaceutical Group Co., Ltd., Moderna, Inc., Serum Institute of India, CureVac AG, CanSino Biologics Inc., Pfizer Inc., Arcturus Therapeutics Holdings Inc., Novavax, Inc., Entos Pharmaceuticals, Sanofi S.A., Symvivo, Glaxo Smith Kline PLC, Daiichi Sankyo Company Ltd., and Astellas Pharma Inc. are the major players in the corona virus vaccine market.
Please note: This is not an exhaustive list of companies profiled in the report.
Chapter 1 Methodology
1.1 Market Scope & Definitions
1.2 Estimates & Forecast Calculation
1.3 Historical Data Overview and Validation
1.4 Data Sources
1.4.1 Secondary
1.4.2 Primary
Chapter 2 Report Outlook
2.1 Corona Virus Vaccine Industry Overview, 2021-2026
2.1.1 Industry Overview
2.1.2 Product Type Overview
2.1.3 Vaccine Type Overview
2.1.4 End-User Overview
2.1.5 Technology Overview
2.1.6 Patient Type Overview
2.1.6 Clinical Trial Phase Overview
2.1.6 Regional Overview
Chapter 3 Corona Virus Vaccine Market Trends
3.1 Market Segmentation
3.2 Industry Background, 2021-2026
3.3 Market Key Trends
3.3.1 Positive Trends
3.3.1.1 Rising COVID-19 cases across the globe
3.3.1.2 Increasing research activities related to producing vaccine in bulk
3.3.2 Industry Challenges
3.3.2.1 Rapidly growing COVID-19 cases of patients with comorbidity issues and lack of government infrastructure for efficient vaccination procedure
3.4 Prospective Growth Scenario
3.4.1 Product Type Growth Scenario
3.4.2 Vaccine Type Growth Scenario
3.4.3 End-User Growth Scenario
3.4.4 Technology Growth Scenario
3.4.5 Patient Type Scenario
3.4.6 Clinical Trial Phase Scenario
3.5 COVID-19 Influence over Industry Growth
3.6 Porter’s Analysis
3.7 PESTEL Analysis
3.8 Value Chain & Supply Chain Analysis
3.9 Regulatory Framework
3.9.1 North America
3.9.2 Europe
3.9.3 APAC
3.9.4 LATAM
3.9.5 MEA
3.10 Technology Overview
3.11 Market Share Analysis, 2020
3.11.1 Company Positioning Overview, 2020
Chapter 4 Corona Virus Vaccine Market, By Product Type
4.1 Product Type Outlook
4.2 Multivalent Vaccine
4.2.1 Market Size, By Region, 2021-2026 (USD Million)
4.3 Monovalent Vaccine
4.3.1 Market Size, By Region, 2021-2026 (USD Million)
Chapter 5 Corona Virus Vaccine Market, By Vaccine Type
5.1 Vaccine Type Outlook
5.2 Inactivated Virus
5.2.1 Market Size, By Region, 2021-2026 (USD Million)
5.3 Protein Subunit
5.3.1 Market Size, By Region, 2021-2026 (USD Million)
5.4 Non-replicating Viral Vector
5.4.1 Market Size, By Region, 2021-2026 (USD Million)
5.5 RNA Vaccine
5.5.1 Market Size, By Region, 2021-2026 (USD Million)
5.6 Other Vaccine Types
5.6.1 Market Size, By Region, 2021-2026 (USD Million)
Chapter 6 Corona Virus Vaccine Market, By End-User
6.1 Government Vaccination Centers
6.1.1 Market Size, By Region, 2021-2026 (USD Million)
6.2 Hospitals & Clinics
6.2.1 Market Size, By Region, 2021-2026 (USD Million)
6.3 E-Pharmacy Channel
6.3.1 Market Size, By Region, 2021-2026 (USD Million)
6.4 Research & Development Organizations
6.4.1 Market Size, By Region, 2021-2026 (USD Million)
6.5 Others
6.5.1 Market Size, By Region, 2021-2026 (USD Million)
Chapter 7 Corona Virus Vaccine Market, By Technology
7.1 Vector-Based
7.1.1 Market Size, By Region, 2021-2026 (USD Million)
7.2 Nucleic Acid-Based
7.2.1 Market Size, By Region, 2021-2026 (USD Million)
7.3 Protein-Based
7.3.1 Market Size, By Region, 2021-2026 (USD Million)
7.4 Whole Virus
7.4.1 Market Size, By Region, 2021-2026 (USD Million)
Chapter 8 Corona Virus Vaccine Market, By Clinical Trial Phase
8.1 Phase 1
8.1.1 Market Size, By Region, 2021-2026 (USD Million)
8.2 Phase 2
8.2.1 Market Size, By Region, 2021-2026 (USD Million)
8.3 Phase 3
8.3.1 Market Size, By Region, 2021-2026 (USD Million)
8.4 Market Launched
8.4.1 Market Size, By Region, 2021-2026 (USD Million)
Chapter 9 Corona Virus Vaccine Market, By Patient Type
9.1 Adult & Geriatric Patients
9.1.1 Market Size, By Region, 2021-2026 (USD Million)
9.2 Pediatric Patients
9.2.1 Market Size, By Region, 2021-2026 (USD Million)
Chapter 10 Corona Virus Vaccine Market, By Region
10.1 Regional outlook
10.2 North America
10.2.1 Market Size, By Country 2021-2026 (USD Million)
10.2.2 Market Size, By Product Type, 2021-2026 (USD Million)
10.2.3 Market Size, By Vaccine Type, 2021-2026 (USD Million)
10.2.4 Market Size, By End-User, 2021-2026 (USD Million)
10.2.5 Market Size, By Technology, 2021-2026 (USD Million)
10.2.6 Market Size, By Clinical Trial, 2021-2026 (USD Million)
10.2.7 Market Size, By Patient Type, 2021-2026 (USD Million)
10.2.8 U.S.
10.2.8.1 Market Size, By Product Type, 2021-2026 (USD Million)
10.2.8.2 Market Size, By Vaccine Type, 2021-2026 (USD Million)
10.2.8.3 Market Size, By End-User, 2021-2026 (USD Million)
10.2.8.4 Market Size, By Technology, 2021-2026 (USD Million)
10.2.8.5 Market Size, By Clinical Trial, 2021-2026 (USD Million)
10.2.8.6 Market Size, By Patient Type, 2021-2026 (USD Million)
10.2.9 Canada
10.2.9.1 Market Size, By Product Type, 2021-2026 (USD Million)
10.2.9.2 Market Size, By Vaccine Type, 2021-2026 (USD Million)
10.2.9.3 Market Size, By End-User, 2021-2026 (USD Million)
10.2.9.4 Market Size, By Technology, 2021-2026 (USD Million)
10.2.9.5 Market Size, By Clinical Trial, 2021-2026 (USD Million)
10.2.9.6 Market Size, By Patient Type, 2021-2026 (USD Million)
10.2.10 Mexico
10.2.10.1 Market Size, By Product Type, 2021-2026 (USD Million)
10.2.10.2 Market Size, By Vaccine Type, 2021-2026 (USD Million)
10.2.10.3 Market Size, By End-User, 2021-2026 (USD Million)
10.2.10.4 Market Size, By Technology, 2021-2026 (USD Million)
10.2.10.5 Market Size, By Clinical Trial, 2021-2026 (USD Million)
10.2.10.6 Market Size, By Patient Type, 2021-2026 (USD Million)
10.3 Europe
10.3.1 Market Size, By Country 2021-2026 (USD Million)
10.3.2 Market Size, By Product Type, 2021-2026 (USD Million)
10.3.3 Market Size, By Vaccine Type, 2021-2026 (USD Million)
10.3.4 Market Size, By End-User, 2021-2026 (USD Million)
10.3.5 Market Size, By Technology, 2021-2026 (USD Million)
10.3.6 Market Size, By Clinical Trial, 2021-2026 (USD Million)
10.3.7 Market Size, By Patient Type, 2021-2026 (USD Million)
10.3.8 Germany
10.3.8.1 Market Size, By Product Type, 2021-2026 (USD Million)
10.3.8.2 Market Size, By Vaccine Type, 2021-2026 (USD Million)
10.3.8.3 Market Size, By End-User, 2021-2026 (USD Million)
10.3.8.4 Market Size, By Technology, 2021-2026 (USD Million)
10.3.8.5 Market Size, By Clinical Trial, 2021-2026 (USD Million)
10.3.8.6 Market Size, By Patient Type, 2021-2026 (USD Million)
10.3.9 UK
10.3.9.1 Market Size, By Product Type, 2021-2026 (USD Million)
10.3.9.2 Market Size, By Vaccine Type, 2021-2026 (USD Million)
10.3.9.3 Market Size, By End-User, 2021-2026 (USD Million)
10.3.9.4 Market Size, By Technology, 2021-2026 (USD Million)
10.3.9.5 Market Size, By Clinical Trial, 2021-2026 (USD Million)
10.3.9.6 Market Size, By Patient Type, 2021-2026 (USD Million)
10.3.10 France
10.3.10.1 Market Size, By Product Type, 2021-2026 (USD Million)
10.3.10.2 Market Size, By Vaccine Type, 2021-2026 (USD Million)
10.3.10.3 Market Size, By End-User, 2021-2026 (USD Million)
10.3.10.4 Market Size, By Technology, 2021-2026 (USD Million)
10.3.10.5 Market Size, By Clinical Trial, 2021-2026 (USD Million)
10.3.10.6 Market Size, By Patient Type, 2021-2026 (USD Million)
10.3.11 Italy
10.3.11.1 Market Size, By Product Type, 2021-2026 (USD Million)
10.3.11.2 Market Size, By Vaccine Type, 2021-2026 (USD Million)
10.3.11.3 Market Size, By End-User, 2021-2026 (USD Million)
10.3.11.4 Market Size, By Technology, 2021-2026 (USD Million)
10.3.11.5 Market Size, By Clinical Trial, 2021-2026 (USD Million)
10.3.11.6 Market Size, By Patient Type, 2021-2026 (USD Million)
10.4 Asia Pacific
10.4.1 Market Size, By Country 2021-2026 (USD Million)
10.4.2 Market Size, By Product Type, 2021-2026 (USD Million)
10.4.3 Market Size, By Vaccine Type, 2021-2026 (USD Million)
10.4.4 Market Size, By End-User, 2021-2026 (USD Million)
10.4.5 Market Size, By Technology, 2021-2026 (USD Million)
10.4.6 Market Size, By Clinical Trial, 2021-2026 (USD Million)
10.4.7 Market Size, By Patient Type, 2021-2026 (USD Million)
10.4.8 China
10.4.8.1 Market Size, By Product Type, 2021-2026 (USD Million)
10.4.8.2 Market Size, By Vaccine Type, 2021-2026 (USD Million)
10.4.8.3 Market Size, By End-User, 2021-2026 (USD Million)
10.4.8.4 Market Size, By Technology, 2021-2026 (USD Million)
10.4.8.5 Market Size, By Clinical Trial, 2021-2026 (USD Million)
10.4.8.6 Market Size, By Patient Type, 2021-2026 (USD Million)
10.4.9 India
10.4.9.1 Market Size, By Product Type, 2021-2026 (USD Million)
10.4.9.2 Market Size, By Vaccine Type, 2021-2026 (USD Million)
10.4.9.3 Market Size, By End-User, 2021-2026 (USD Million)
10.4.9.4 Market Size, By Technology, 2021-2026 (USD Million)
10.4.9.5 Market Size, By Clinical Trial, 2021-2026 (USD Million)
10.4.9.6 Market Size, By Patient Type, 2021-2026 (USD Million)
10.4.10 Japan
10.4.8.1 Market Size, By Product Type, 2021-2026 (USD Million)
10.4.8.2 Market Size, By Vaccine Type, 2021-2026 (USD Million)
10.4.8.3 Market Size, By End-User, 2021-2026 (USD Million)
10.4.8.4 Market Size, By Technology, 2021-2026 (USD Million)
10.4.8.5 Market Size, By Clinical Trial, 2021-2026 (USD Million)
10.4.8.6 Market Size, By Patient Type, 2021-2026 (USD Million)
10.5 MEA
10.5.1 Market Size, By Country 2021-2026 (USD Million)
10.5.2 Market Size, By Product Type, 2021-2026 (USD Million)
10.5.3 Market Size, By Vaccine Type, 2021-2026 (USD Million)
10.5.4 Market Size, By End-User, 2021-2026 (USD Million)
10.5.5 Market Size, By Technology, 2021-2026 (USD Million)
10.5.6 Market Size, By Clinical Trial, 2021-2026 (USD Million)
10.5.7 Market Size, By Patient Type, 2021-2026 (USD Million)
10.5.8 Saudi Arabia
10.5.8.1 Market Size, By Product Type, 2021-2026 (USD Million)
10.5.8.2 Market Size, By Vaccine Type, 2021-2026 (USD Million)
10.5.8.3 Market Size, By End-User, 2021-2026 (USD Million)
10.5.8.4 Market Size, By Technology, 2021-2026 (USD Million)
10.5.8.5 Market Size, By Clinical Trial, 2021-2026 (USD Million)
10.5.8.6 Market Size, By Patient Type, 2021-2026 (USD Million)
10.5.9 UAE
10.5.9.1 Market Size, By Product Type, 2021-2026 (USD Million)
10.5.9.2 Market Size, By Vaccine Type, 2021-2026 (USD Million)
10.5.9.3 Market Size, By End-User, 2021-2026 (USD Million)
10.5.9.4 Market Size, By Technology, 2021-2026 (USD Million)
10.5.9.5 Market Size, By Clinical Trial, 2021-2026 (USD Million)
10.5.9.6 Market Size, By Patient Type, 2021-2026 (USD Million)
10.5.10 South Africa
10.5.10.1 Market Size, By Product Type, 2021-2026 (USD Million)
10.5.10.2 Market Size, By Vaccine Type, 2021-2026 (USD Million)
10.5.10.3 Market Size, By End-User, 2021-2026 (USD Million)
10.5.10.4 Market Size, By Technology, 2021-2026 (USD Million)
10.5.10.5 Market Size, By Clinical Trial, 2021-2026 (USD Million)
10.5.10.6 Market Size, By Patient Type, 2021-2026 (USD Million)
Chapter 11 Company Landscape
11.1 Competitive Analysis, 2020
11.2 Pfizer
11.2.1 Company Overview
11.2.2 Financial Analysis
11.2.3 Strategic Positioning
11.2.4 Info Graphic Analysis
11.3 AstraZaneca
11.3.1 Company Overview
11.3.2 Financial Analysis
11.3.3 Strategic Positioning
11.3.4 Info Graphic Analysis
11.4 Johnson and Johnson
11.4.1 Company Overview
11.4.2 Financial Analysis
11.4.3 Strategic Positioning
11.4.4 Info Graphic Analysis
11.5 Bharat Biotech International Limited
11.5.1 Company Overview
11.5.2 Financial Analysis
11.5.3 Strategic Positioning
11.5.4 Info Graphic Analysis
11.6 Sinovac Biotech Ltd.,
11.6.1 Company Overview
11.6.2 Financial Analysis
11.6.3 Strategic Positioning
11.6.4 Info Graphic Analysis
11.7 Serum Institute
11.7.1 Company Overview
11.7.2 Financial Analysis
11.7.3 Strategic Positioning
11.7.4 Info Graphic Analysis
11.8 BioNTech SE
11.10.1 Company Overview
11.10.2 Financial Analysis
11.10.3 Strategic Positioning
11.10.4 Info Graphic Analysis
11.11 Cadila Healthcare Limited
11.11.1 Company Overview
11.11.2 Financial Analysis
11.11.3 Strategic Positioning
11.11.4 Info Graphic Analysis
11.10 Others
11.10.1 Company Overview
11.10.2 Financial Analysis
11.10.3 Strategic Positioning
11.10.4 Info Graphic Analysis
The Global Corona Virus Vaccine Market has been studied from the year 2019 till 2026. However, the CAGR provided in the report is from the year 2021 to 2026. The research methodology involved three stages: Desk research, Primary research, and Analysis & Output from the entire research process.

The desk research involved a robust background study which meant referring to paid and unpaid databases to understand the market dynamics; mapping contracts from press releases; identifying the key players in the market, studying their product portfolio, competition level, annual reports/SEC filings & investor presentations; and learning the demand and supply-side analysis for the Corona Virus Vaccine Market.

The primary research activity included telephonic conversations with more than 50 tier 1 industry consultants, distributors, and end-use product manufacturers.

Finally, based on the above thorough research process, an in-depth analysis was carried out considering the following aspects: market attractiveness, current & future market trends, market share analysis, SWOT analysis of the company and customer analytics.

Tailor made solutions just for you
80% of our clients seek made-to-order reports. How do you want us to tailor yours?
OUR CLIENTS