
Global Interventional Cardiology Devices Market Size, Trends, and Analysis - Forecasts To 2026 By Product (Angioplasty Balloons, Angioplasty Stents, Structural Heart Devices, Catheters, Plaque Modification Devices, Hemodynamic Flow Alteration Devices, Other Devices), By Region (North America, Asia Pacific, CSA, Europe, and the Middle East and Africa); End-User Landscape, Company Market Share Analysis & Competitor Analysis
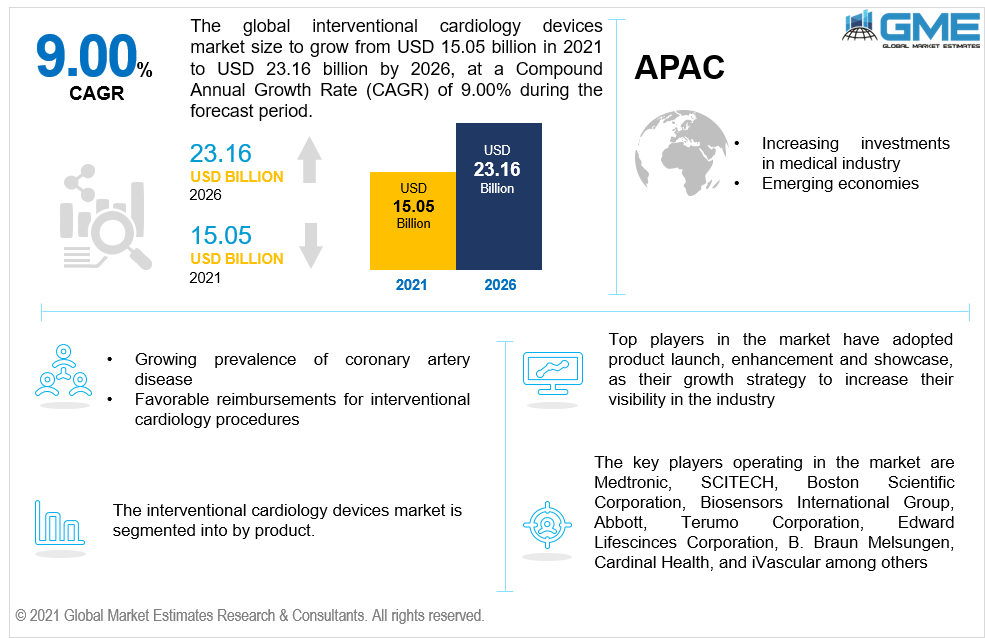
Interventional cardiology is a branch of the cardiology department that is involved in using catheters for the treatment of heart diseases. The devices are widely used for treating cardiovascular diseases. The global Interventional cardiology devices market will register a peak growth rate and is expected to follow the trend between 2021-2026, owing to a high prevalence of coronary artery diseases. The increasing tobacco consumption globally, increasing number obesity patients, and favorable reimbursement activities are the primary factors supporting the market growth. Rapid growth in the global population and the rising incidence of diabetes among all age groups are boosting the market growth. According to the facts published by the British Heart Foundation, it was estimated that more than 2.30 million people are suffering from CAD in the UK. About 170,000 people in the UK losing their lives every year due to heart and circulatory diseases. The growing adoption of minimally invasive surgeries and the introduction and adoption of advanced medical devices are also propelling the market growth. However, the availability of alternative medical treatments and product failures and recalls are hampering the global market growth.
Based on the product, the market is analyzed and categorized into angioplasty balloons, angioplasty stents, structural heart devices, catheters, plaque modification devices, and hemodynamic flow alteration devices among others. The hemodynamic flow alteration devices segment dominated the global market and hold the largest share between 2021-2026. The segment is furthered explained into embolic protection devices and chronic total occlusion devices. Embolic protection devices capture embolic debris without disturbing continuous blood flow. The growing adoption of interventional procedures among the medical sector to treat coronary artery diseases are driving the segmental growth
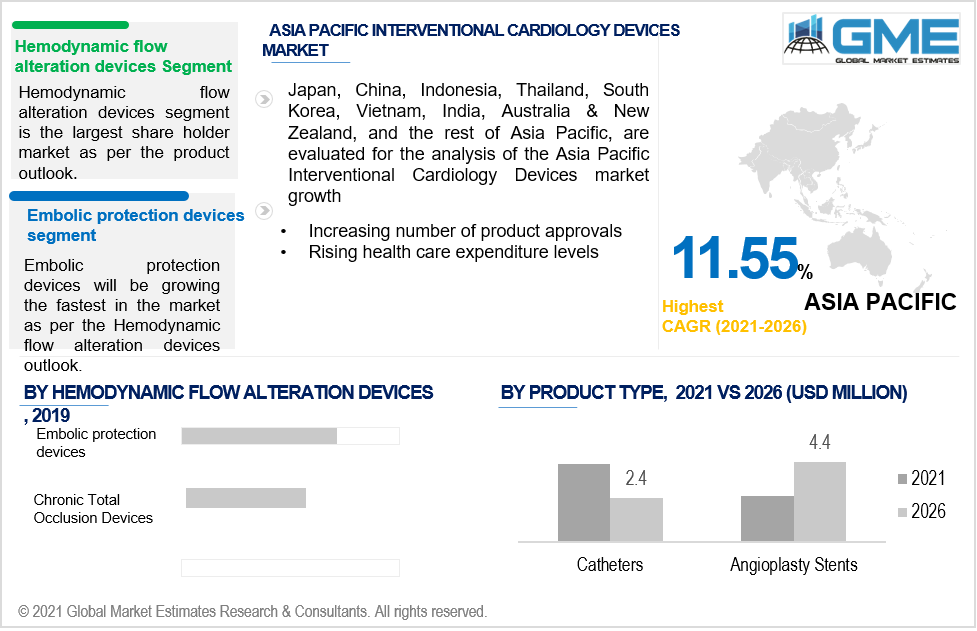
According to GME analysis, the North American region will be the leading region and occupies a major chunk of the market, owing to rapid growth in the aged population and high prevalence of diabetics. The presence of prominent medical device players such as Medtronic, Abbott, Cardinal Health, and Boston Scientific Corporation among others are also supporting the market growth. Favorable government reimbursement programs and the presence of good medical infrastructure in the U.S and Canada are supporting the region's growth.
Key players operating in the market include are Medtronic, SCITECH, Boston Scientific Corporation, Biosensors International Group, Abbott, Terumo Corporation, Edward Lifesciences Corporation, B. Braun Melsungen, Cardinal Health, vascular, BIOTRONIK SE & Co. KG, Becton, ENDOCOR GMBH, Dickinson, and Company, Insitu Technologies, Inc., Meril Life Sciences Pvt. Ltd., Alvimedica, Cardionovum Gmbh, Smt, Medinol Ltd., and Wellinq among others.
Please note: This is not an exhaustive list of companies profiled in the report.
We value your investment and offer free customization with every report to fulfil your exact research needs.
The Global Interventional Cardiology Devices Market has been studied from the year 2019 till 2026. However, the CAGR provided in the report is from the year 2021 to 2026. The research methodology involved three stages: Desk research, Primary research, and Analysis & Output from the entire research process.

The desk research involved a robust background study which meant referring to paid and unpaid databases to understand the market dynamics; mapping contracts from press releases; identifying the key players in the market, studying their product portfolio, competition level, annual reports/SEC filings & investor presentations; and learning the demand and supply side analysis for the Interventional Cardiology Devices Market.

The primary research activity included telephonic conversations with more than 50 tier 1 industry consultants, distributors, and end-use product manufacturers.

Finally, based on the above thorough research process, an in-depth analysis was carried out considering the following aspects: market attractiveness, current & future market trends, market share analysis, SWOT analysis of the companies and customer analytics.

Tailor made solutions just for you
80% of our clients seek made-to-order reports. How do you want us to tailor yours?
OUR CLIENTS