
Global Pyrogen Testing Market Size, Trends and Analysis - Forecasts to 2026 By Product & Services (Assays, Kits & Reagents, Instruments, Services), By Test Type (LAL Tests, In Vitro Tests, Rabbit Tests), By End-User (Pharmaceutical & Biotechnology Companies, Medical Device Companies, Other End-User), By Region (North America, Asia-Pacific, Middle East & Africa, CSA, Europe), End-User Landscape and Company Market Share Analysis and Competitor Analysis
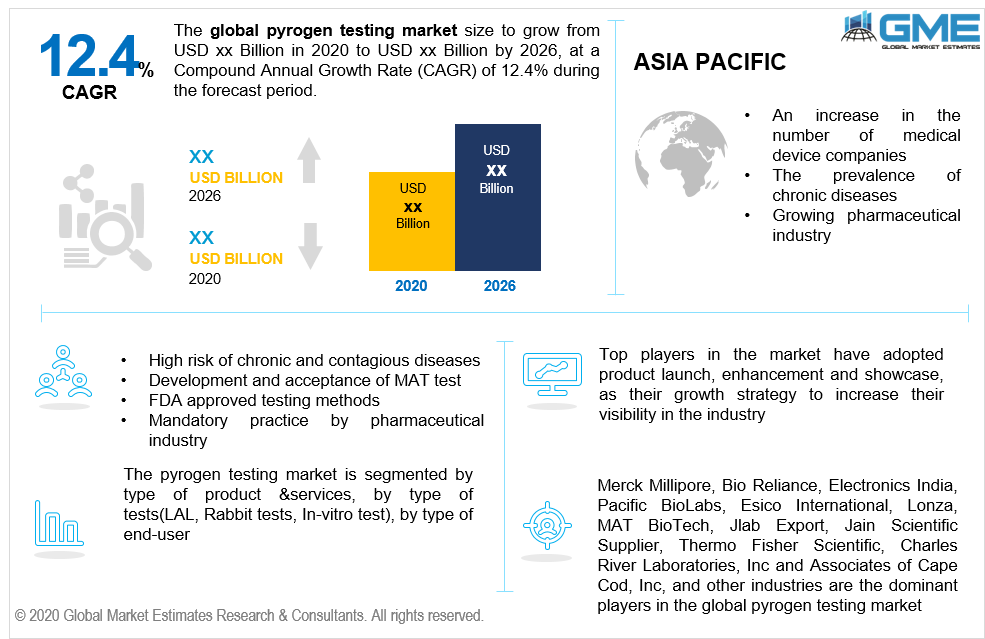
The pyrogen testing market will follow an upward rising trend during the forecast period. Pyrogens are substances that cause fever in humans as well as animals which are attained from either lipopolysaccharide and endotoxins. The high quantity of such microorganisms can lead to multiorgan failure, shock, and inflammation. High risk of contagious diseases, seasonal and weather variations, and appropriate guidelines in place for pyrogen testing enhance the performance of the market. The development of newer methods ( MAT) which exploits human cells as an alternative to the Rabbit test is fast gaining momentum in the market space as the test is relatively faster and equipped to detects pyrogen better than the Rabbit test. Such advances and developments overall lead to market enhancement during the forecast period. Wide acceptance of the MAT test in the European region and the approval by the FDA authorities enhance the market performance during the forecast period. The COVID-19 situation has also led the pharmaceutical and biotechnology companies to develop new vaccines and detectable methods to fight off bacteria and microorganisms causing fever making pyrogen testing to remain competitive in the market domain.
Strict and mandatory pyrogen detection by the pharmaceutical industry and industry-wide application of the pyrogen testing method in medical devices, biologicals, pharmaceutical, and cell therapeutics firmly shape the market demand in the aforementioned period.
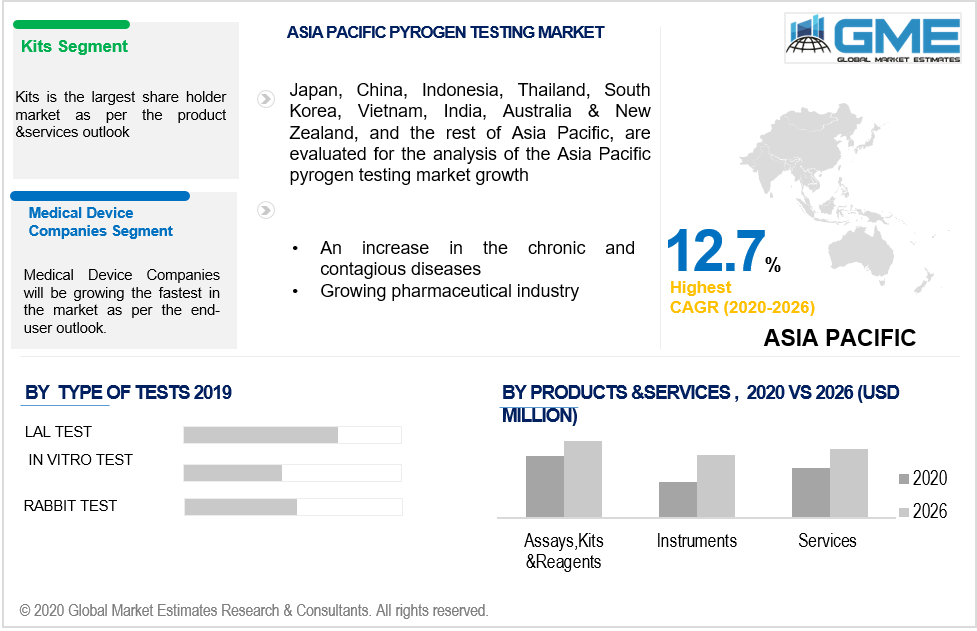
Based on the products and services, the market is segmented into reagents, kits, services, assays, and instruments. Kits domain would be gearing up the market demand for the pyrogen testing. The PyroMAT testing kits are preferred over the other methods of testing due to varied benefits. It offers patients safety, highly flexible than the Rabbit testing or LAL, is in accord with the FDA, and other international guidelines and regulations, and is also capable of replacing animal testing through the in vitro methods. Apart from such advantages, the PyroMat kit is designed such that it does not require competencies or cell culture equipment and also ensures patient safety. These advantages add to the growth of the market in the coming years. The reagents are extensively used for the testing of medical devices. Given robust applications and advantages of reagent, kits and assays make the segment accelerate the market demand in the given timeframe.
Based on the test type, the market studies in-vitro, LAL, and rabbit tests into being. The LAL tests will have a large market penetration owing to the test’s ability to replace the rabbit testing and follow standard regulations prevalent in the pharmaceutical industry. The LAL test ensures the safety of sterile pharmaceutical devices and products. The sterile pharmaceutical drugs and products have risen in the few years owing to rising hospital infections, pandemics, and infectious diseases. Such factors lead to the high deployment of the LAL tests in the market domain to ensure maximum safety.
Based on the end-user, the pharmaceutical and biotechnology companies will be a dominant player in the market but medical devices companies will emerge as the fastest-growing domain in the aforementioned period. Endotoxins are pyrogen present in medical devices and can cause extreme risk to human health if left undetected. The LAL test enables the safety of medical devices and also offers medical device companies in adhering to the standard protocol in conducting pre-pyrogen testing.
Given the regional study, the European region would be a prominent player in the pyrogen testing market. The incorporation of MAT test in the EU Pharmacopoeia, EU legislation approve in-vitro test, presence of key medical companies and prominent pharmaceutical industry in the region would place the region on a competitive front and accentuate the market growth.
Key players operating in the market include Merck Millipore, BioReliance, Electronics India, Pacific BioLabs, Esico International, Lonza, MAT BioTech, Jlab Export, Jain Scientific Supplier, Thermo Fisher Scientific, Charles River Laboratories, Inc and Associates of Cape Cod, Inc. among others
Please note: This is not an exhaustive list of companies profiled in the report.
In May 2020, Lonza partnered with Sanquin to commercialize its specialized reagents by using MAT tests.
We value your investment and offer free customization with every report to fulfil your exact research needs.
The Global Pyrogen Testing Market has been studied from the year 2017 till 2026. However, the CAGR provided in the report is from the year 2018 to 2026. The research methodology involved three stages: Desk research, Primary research, and Analysis & Output from the entire research process.

The desk research involved a robust background study which meant referring to paid and unpaid databases to understand the market dynamics; mapping contracts from press releases; identifying the key players in the market, studying their product portfolio, competition level, annual reports/SEC filings & investor presentations; and learning the demand and supply side analysis for the Pyrogen Testing Market.

The primary research activity included telephonic conversations with more than 50 tier 1 industry consultants, distributors, and end-use product manufacturers.

Finally, based on the above thorough research process, an in-depth analysis was carried out considering the following aspects: market attractiveness, current & future market trends, market share analysis, SWOT analysis of the companies and customer analytics.

Tailor made solutions just for you
80% of our clients seek made-to-order reports. How do you want us to tailor yours?
OUR CLIENTS