
Global Medical Device Package Validation Market Size, Trends & Analysis - Forecasts to 2029 By Testing Type (Physical Testing, Microbial Testing, Chemical Testing, and Visual Testing), By Device Class (Class I Devices, Class II Devices, and Class III Devices), and By Region (North America, Asia Pacific, Central and South America, Europe, and Middle East and Africa), Competitive Landscape, Company Market Share Analysis, and End User Analysis
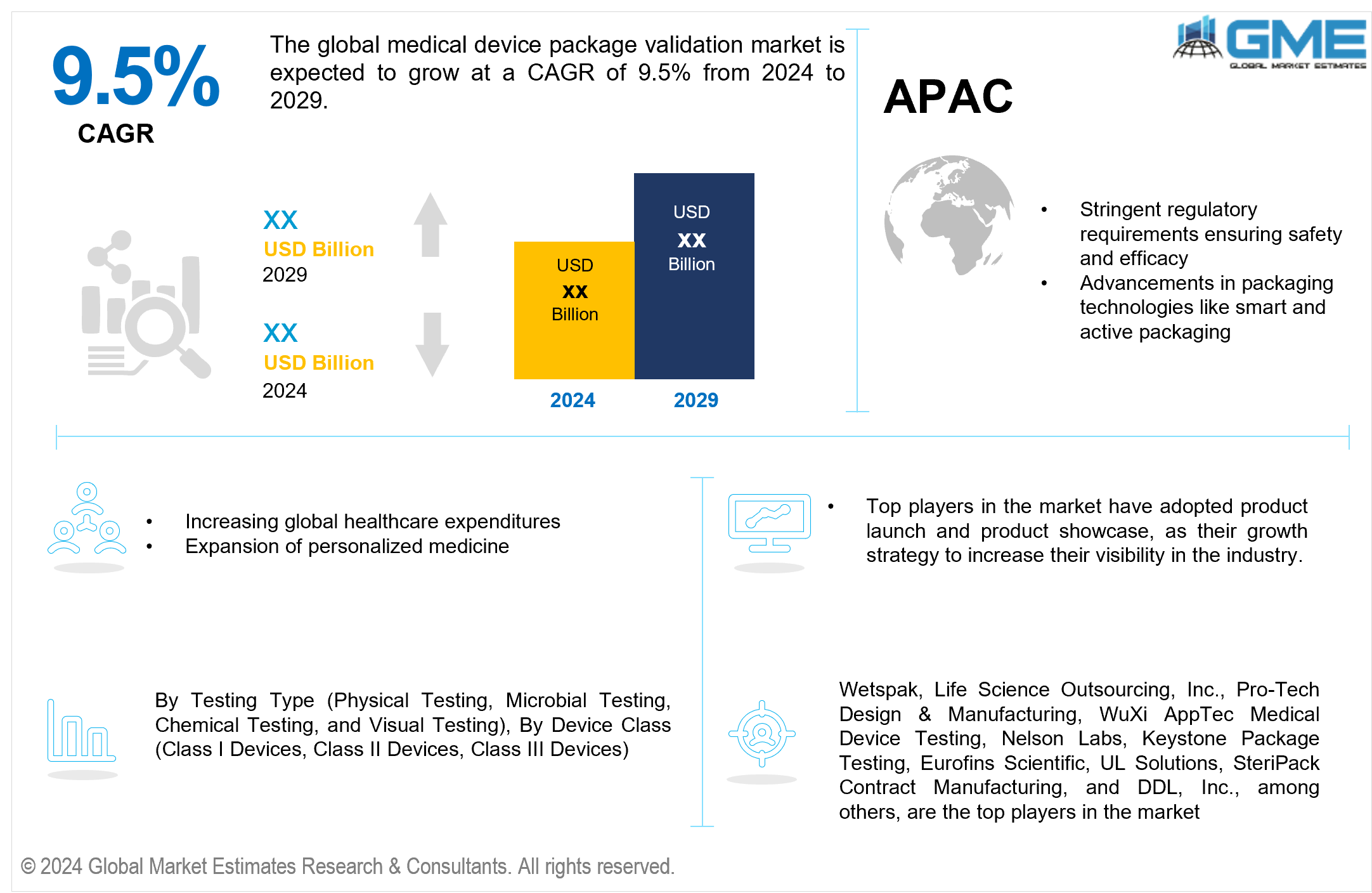
The global medical device package validation market is expected to exhibit a CAGR of 9.5% from 2024 to 2029.
The medical device package validation market is driven by stringent regulatory requirements, ensuring the safety, efficacy, and integrity of medical devices throughout their lifecycle. Regulatory bodies such as the FDA and CE mark necessitate thorough validation processes to mitigate risks associated with packaging failures, ensuring products remain sterile and safe for patient use. This regulatory scrutiny compels medical device manufacturers to invest in robust package validation solutions that comply with international standards, thereby driving market growth.
Moreover, advancements in packaging materials and technologies play a pivotal role in shaping the market landscape. Innovations such as active and intelligent packaging systems, capable of monitoring and maintaining product integrity during storage and transportation, are gaining traction. These technologies enhance packaging performance and contribute to extended shelf life and improved product safety. Additionally, the rise of personalized medicine and biologics necessitates packaging solutions that can accommodate complex and sensitive materials, further fueling demand for advanced validation techniques. As the medical device industry continues to innovate and expand globally, the package validation market is expected to grow, driven by the imperative to ensure compliance, reliability, and patient safety across diverse healthcare settings.
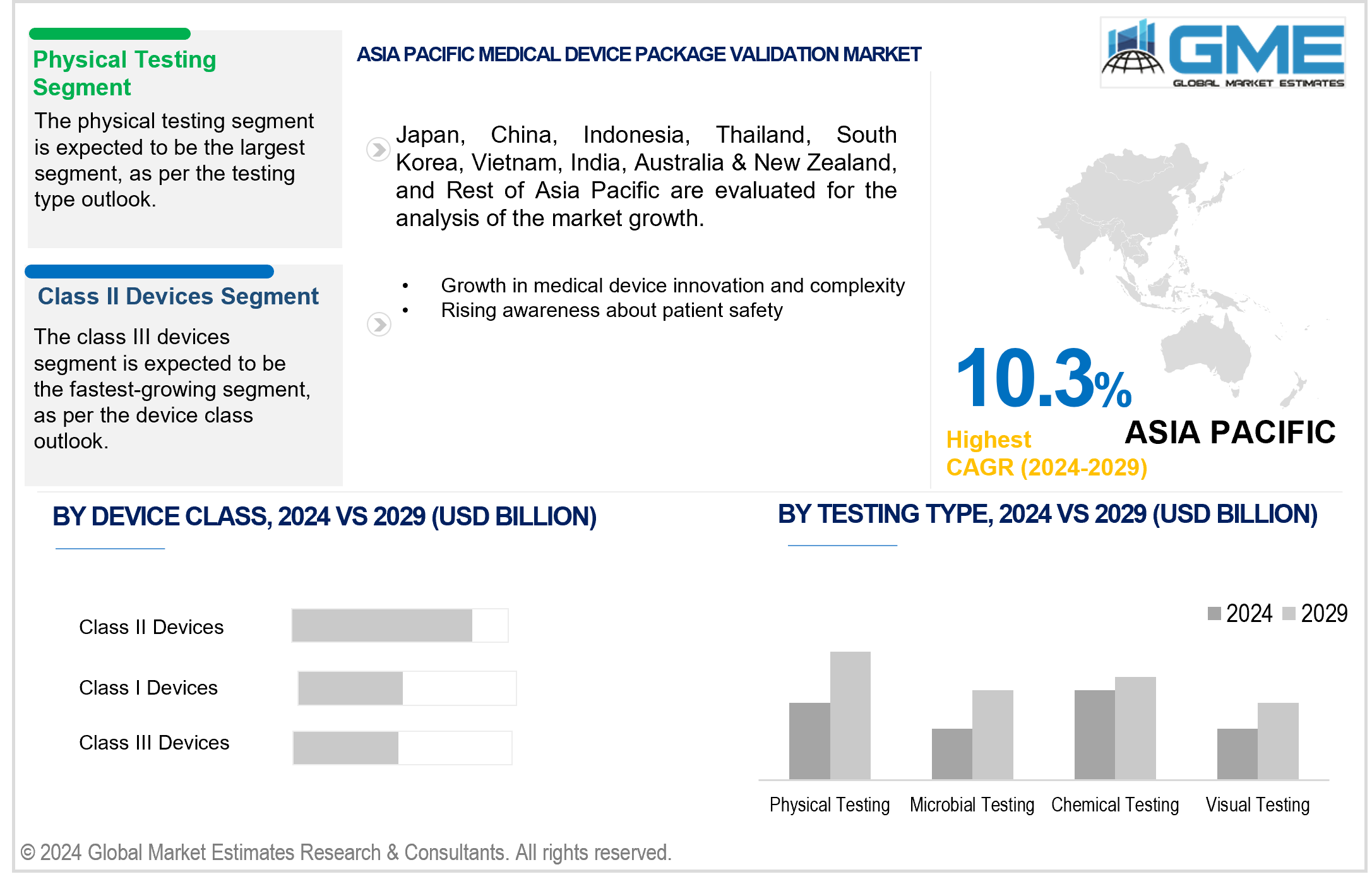
In the medical device package validation market, physical testing is analyzed to be the largest segment by testing type. Physical testing involves assessing the mechanical properties, durability, and integrity of packaging materials and systems under various conditions. This includes tests for compression, vibration, and impact resistance to ensure packages can withstand the rigors of distribution and storage without compromising the sterility and functionality of medical devices. The demand for robust physical testing solutions is driven by regulatory requirements and the need to prevent package failures that could jeopardize patient safety and product efficacy.
Conversely, microbial testing is anticipated to be the fastest-growing segment in the medical device package validation market. With the increasing focus on infection control and sterility assurance, microbial testing plays a critical role in evaluating the effectiveness of packaging materials in preventing microbial ingress and maintaining sterility throughout the device's shelf life. This testing type includes assessments for bacterial, fungal, and viral contamination, ensuring compliance with stringent regulatory standards and enhancing patient safety.
Class II devices are expected to emerge as the largest segment in terms of package validation requirements. Class II devices, which include moderate-risk medical devices such as infusion pumps and diagnostic imaging equipment, require thorough validation to ensure packaging maintains sterility and product integrity. The complexity and variability of Class II devices necessitate comprehensive testing protocols, driving demand for tailored validation solutions that meet regulatory expectations and industry standards.
On the other hand, class III devices represent the fastest-growing segment in the medical device package validation market by device class. These high-risk devices, such as implantable devices and life-supporting equipment, demand stringent validation processes to mitigate risks associated with packaging failures. The critical nature of Class III devices underscores the importance of advanced testing methodologies, including physical, chemical, and microbial assessments, to uphold product safety and regulatory compliance. As advancements in medical technology continue to evolve, the package validation market for Class III devices is expected to expand, driven by the imperative to ensure reliability and efficacy in highly sensitive medical applications.
North America is expected to be the largest region in the global medical device package validation market. The market's dominance is attributed to its robust healthcare infrastructure, stringent regulatory frameworks such as FDA requirements, and the concentration of leading medical device manufacturers. These factors drive extensive demand for comprehensive package validation services across the region, ensuring compliance with rigorous quality and safety standards. Additionally, ongoing advancements in medical technology and the presence of major market players further bolster the growth of package validation solutions in North America.
Asia Pacific is anticipated to emerge as the fastest-growing region in the global medical device package validation market. The regional market's rapid growth is fueled by expanding healthcare expenditures, increasing adoption of medical devices, and rising awareness about patient safety and regulatory compliance. Countries such as China, India, and Japan are witnessing significant investments in healthcare infrastructure and manufacturing capabilities, driving demand for reliable package validation services. Moreover, initiatives to harmonize regulatory standards across APAC countries and the growing focus on quality assurance in medical device manufacturing contribute to the region's accelerated growth of package validation solutions. As healthcare systems in APAC continue to evolve and modernize, the demand for stringent package validation processes is expected to escalate, driving APAC market growth.
Wetspak, Life Science Outsourcing, Inc., Pro-Tech Design & Manufacturing, WuXi AppTec Medical Device Testing, Nelson Labs, Keystone Package Testing, Eurofins Scientific, UL Solutions, SteriPack Contract Manufacturing, and DDL, Inc., among others, are the top players in the market.
Please note: This is not an exhaustive list of companies profiled in the report.
1 STRATEGIC INSIGHTS ON NEW REVENUE POCKETS
1.1 Strategic Opportunity & Attractiveness Analysis
1.1.1 Hot Revenue Pockets
1.1.2 Market Attractiveness Score
1.1.3 Revenue Impacting Opportunity
1.1.4 High Growing Region/Country
1.1.5 Competitor Analysis
1.1.6 Consumer Analysis
1.2 Global Market Estimates' View
1.3 Strategic Insights across Business Functions
1.3.1 For Chief Executive Officers
1.3.2 For Chief Marketing Officers
1.3.3 For Chief Strategy Officers
1.4 Evaluate the Potential of your Existing Business Lines vs. New Lines to Enter Into
2 TECHNOLOGICAL TRENDS
2.1 Technological Adoption Rate
2.2 Current Trend Impact Analysis
2.3 Future Trend Impact Analysis
3 GLOBAL MARKET OUTLOOK
3.1 Market Pyramid Analysis
3.1.1 Introduction
3.1.2 Adjacent Market Opportunities
3.1.3 Ancillary Market Opportunities
3.2 Demand Side Analysis
3.2.1 Market Drivers: Impact Analysis
3.2.2 Market Restraints: Impact Analysis
3.2.3 Market Opportunities: Impact Analysis
3.2.4 Market Challenges: Impact Analysis
3.3 Supply Side Analysis
3.3.1 Porter’s Five Forces Analysis
3.3.1.1 Threat of New Entrants
3.3.1.2 Threat of New Substitutes
3.3.1.3 Bargaining Power of Suppliers
3.3.1.4 Bargaining Power of Buyers
3.3.1.5 Intensity of Competitive Rivalry
3.3.2 SWOT Analysis; By Factor (Political & Legal, Economic, and Technological)
3.3.2.1 Political Landscape
3.3.2.2 Economic Landscape
3.3.2.3 Social Landscape
3.3.2.4 Technology Landscape
3.3.3 Value Chain Analysis
3.3.4 Trend Analysis
3.3.5 Gap Analysis
3.3.6 Cost Analysis
4 GLOBAL MEDICAL DEVICE PACKAGE VALIDATION MARKET, BY TESTING TYPE
4.1 Introduction
4.2 Medical Device Package Validation Market: Testing Type Scope Key Takeaways
4.3 Revenue Growth Analysis, 2023 & 2029
4.4 Competitive Analysis
4.4.1 Competitive Analysis Market Estimates and Forecast, 2021-2029 (USD Million)
4.5 Physical Testing
4.5.1 Physical Testing Market Estimates and Forecast, 2021-2029 (USD Million)
4.6 Microbial Testing
4.6.1 Microbial Testing Market Estimates and Forecast, 2021-2029 (USD Million)
4.7 Chemical Testing
4.7.1 Chemical Testing Market Estimates and Forecast, 2021-2029 (USD Million)
4.8 Visual Testing
4.8.1 Visual Testing Market Estimates and Forecast, 2021-2029 (USD Million)
5 GLOBAL MEDICAL DEVICE PACKAGE VALIDATION MARKET, BY DEVICE CLASS
5.1 Introduction
5.2 Medical Device Package Validation Market: Device Class Scope Key Takeaways
5.3 Revenue Growth Analysis, 2023 & 2029
5.4 Class I Devices
5.4.1 Class I Devices Market Estimates and Forecast, 2021-2029 (USD Million)
5.5 Class II Devices
5.5.1 Class II Devices Market Estimates and Forecast, 2021-2029 (USD Million)
5.6 Class III Devices
5.6.1 Class III Devices Market Estimates and Forecast, 2021-2029 (USD Million)
6 GLOBAL MEDICAL DEVICE PACKAGE VALIDATION MARKET, BY REGION
6.1 Introduction
6.2 North America Medical Device Package Validation Market Estimates and Forecast, 2021-2029 (USD Million)
6.2.1 By Testing Type
6.2.2 By Device Class
6.2.3 By Country
6.2.3.1 U.S. Medical Device Package Validation Market Estimates and Forecast, 2021-2029 (USD Million)
6.2.3.1.1 By Testing Type
6.2.3.1.2 By Device Class
6.2.3.2 Canada Medical Device Package Validation Market Estimates and Forecast, 2021-2029 (USD Million)
6.2.3.2.1 By Testing Type
6.2.3.2.2 By Device Class
6.2.3.3 Mexico Medical Device Package Validation Market Estimates and Forecast, 2021-2029 (USD Million)
6.2.3.3.1 By Testing Type
6.2.3.3.2 By Device Class
6.3 Europe Medical Device Package Validation Market Estimates and Forecast, 2021-2029 (USD Million)
6.3.1 By Testing Type
6.3.2 By Device Class
6.3.3 By Country
6.3.3.1 Germany Medical Device Package Validation Market Estimates and Forecast, 2021-2029 (USD Million)
6.3.3.1.1 By Testing Type
6.3.3.1.2 By Device Class
6.3.3.2 U.K. Medical Device Package Validation Market Estimates and Forecast, 2021-2029 (USD Million)
6.3.3.2.1 By Testing Type
6.3.3.2.2 By Device Class
6.3.3.3 France Medical Device Package Validation Market Estimates and Forecast, 2021-2029 (USD Million)
6.3.3.3.1 By Testing Type
6.3.3.3.2 By Device Class
6.3.3.4 Italy Medical Device Package Validation Market Estimates and Forecast, 2021-2029 (USD Million)
6.3.3.4.1 By Testing Type
6.3.3.4.2 By Device Class
6.3.3.5 Spain Medical Device Package Validation Market Estimates and Forecast, 2021-2029 (USD Million)
6.3.3.5.1 By Testing Type
6.3.3.5.2 By Device Class
6.3.3.6 Netherlands Medical Device Package Validation Market Estimates and Forecast, 2021-2029 (USD Million)
6.3.3.6.1 By Testing Type
6.3.3.6.2 By Device Class
6.3.3.7 Rest of Europe Medical Device Package Validation Market Estimates and Forecast, 2021-2029 (USD Million)
6.3.3.6.1 By Testing Type
6.3.3.6.2 By Device Class
6.4 Asia Pacific Medical Device Package Validation Market Estimates and Forecast, 2021-2029 (USD Million)
6.4.1 By Testing Type
6.4.2 By Device Class
6.4.3 By Country
6.4.3.1 China Medical Device Package Validation Market Estimates and Forecast, 2021-2029 (USD Million)
6.4.3.1.1 By Testing Type
6.4.3.1.2 By Device Class
6.4.3.2 Japan Medical Device Package Validation Market Estimates and Forecast, 2021-2029 (USD Million)
6.4.3.2.1 By Testing Type
6.4.3.2.2 By Device Class
6.4.3.3 India Medical Device Package Validation Market Estimates and Forecast, 2021-2029 (USD Million)
6.4.3.3.1 By Testing Type
6.4.3.3.2 By Device Class
6.4.3.4 South Korea Medical Device Package Validation Market Estimates and Forecast, 2021-2029 (USD Million)
6.4.3.4.1 By Testing Type
6.4.3.4.2 By Device Class
6.4.3.5 Singapore Medical Device Package Validation Market Estimates and Forecast, 2021-2029 (USD Million)
6.4.3.5.1 By Testing Type
6.4.3.5.2 By Device Class
6.4.3.6 Malaysia Medical Device Package Validation Market Estimates and Forecast, 2021-2029 (USD Million)
6.4.3.6.1 By Testing Type
6.4.3.6.2 By Device Class
6.4.3.7 Thailand Medical Device Package Validation Market Estimates and Forecast, 2021-2029 (USD Million)
6.4.3.6.1 By Testing Type
6.4.3.6.2 By Device Class
6.4.3.8 Indonesia Medical Device Package Validation Market Estimates and Forecast, 2021-2029 (USD Million)
6.4.3.7.1 By Testing Type
6.4.3.7.2 By Device Class
6.4.3.9 Vietnam Medical Device Package Validation Market Estimates and Forecast, 2021-2029 (USD Million)
6.4.3.8.1 By Testing Type
6.4.3.8.2 By Device Class
6.4.3.10 Taiwan Medical Device Package Validation Market Estimates and Forecast, 2021-2029 (USD Million)
6.4.3.10.1 By Testing Type
6.4.3.10.2 By Device Class
6.4.3.11 Rest of Asia Pacific Medical Device Package Validation Market Estimates and Forecast, 2021-2029 (USD Million)
6.4.3.11.1 By Testing Type
6.4.3.11.2 By Device Class
6.5 Middle East and Africa Medical Device Package Validation Market Estimates and Forecast, 2021-2029 (USD Million)
6.5.1 By Testing Type
6.5.2 By Device Class
6.5.3 By Country
6.5.3.1 Saudi Arabia Medical Device Package Validation Market Estimates and Forecast, 2021-2029 (USD Million)
6.5.3.1.1 By Testing Type
6.5.3.1.2 By Device Class
6.5.3.2 U.A.E. Medical Device Package Validation Market Estimates and Forecast, 2021-2029 (USD Million)
6.5.3.2.1 By Testing Type
6.5.3.2.2 By Device Class
6.5.3.3 Israel Medical Device Package Validation Market Estimates and Forecast, 2021-2029 (USD Million)
6.5.3.3.1 By Testing Type
6.5.3.3.2 By Device Class
6.5.3.4 South Africa Medical Device Package Validation Market Estimates and Forecast, 2021-2029 (USD Million)
6.5.3.4.1 By Testing Type
6.5.3.4.2 By Device Class
6.5.3.5 Rest of Middle East and Africa Medical Device Package Validation Market Estimates and Forecast, 2021-2029 (USD Million)
6.5.3.5.1 By Testing Type
6.5.3.5.2 By Device Class
6.6 Central and South America Medical Device Package Validation Market Estimates and Forecast, 2021-2029 (USD Million)
6.6.1 By Testing Type
6.6.2 By Device Class
6.6.3 By Country
6.6.3.1 Brazil Medical Device Package Validation Market Estimates and Forecast, 2021-2029 (USD Million)
6.6.3.1.1 By Testing Type
6.6.3.1.2 By Device Class
6.6.3.2 Argentina Medical Device Package Validation Market Estimates and Forecast, 2021-2029 (USD Million)
6.6.3.2.1 By Testing Type
6.6.3.2.2 By Device Class
6.6.3.3 Chile Medical Device Package Validation Market Estimates and Forecast, 2021-2029 (USD Million)
6.6.3.3.1 By Testing Type
6.6.3.3.2 By Device Class
6.6.3.3 Rest of Central and South America Medical Device Package Validation Market Estimates and Forecast, 2021-2029 (USD Million)
6.6.3.3.1 By Testing Type
6.6.3.3.2 By Device Class
7 COMPETITIVE LANDCAPE
7.1 Company Market Share Analysis
7.2 Four Quadrant Positioning Matrix
7.2.1 Market Leaders
7.2.2 Market Visionaries
7.2.3 Market Challengers
7.2.4 Niche Market Players
7.3 Vendor Landscape
7.3.1 North America
7.3.2 Europe
7.3.3 Asia Pacific
7.3.4 Rest of the World
7.4 Company Profiles
7.4.1 Wetspak
7.4.1.1 Business Description & Financial Analysis
7.4.1.2 SWOT Analysis
7.4.1.3 Products & Services Offered
7.4.1.4 Strategic Alliances between Business Partners
7.4.2 Life Science Outsourcing, Inc.
7.4.2.1 Business Description & Financial Analysis
7.4.2.2 SWOT Analysis
7.4.2.3 Products & Services Offered
7.4.2.4 Strategic Alliances between Business Partners
7.4.3 Pro-Tech Design & Manufacturing
7.4.3.1 Business Description & Financial Analysis
7.4.3.2 SWOT Analysis
7.4.3.3 Products & Services Offered
7.4.3.4 Strategic Alliances between Business Partners
7.4.4 WuXi AppTec Medical Device Testing
7.4.4.1 Business Description & Financial Analysis
7.4.4.2 SWOT Analysis
7.4.4.3 Products & Services Offered
7.4.4.4 Strategic Alliances between Business Partners
7.4.5 Nelson Labs
7.4.5.1 Business Description & Financial Analysis
7.4.5.2 SWOT Analysis
7.4.5.3 Products & Services Offered
7.4.5.4 Strategic Alliances between Business Partners
7.4.6 Keystone Package Testing
7.4.6.1 Business Description & Financial Analysis
7.4.6.2 SWOT Analysis
7.4.6.3 Products & Services Offered
7.4.6.4 Strategic Alliances between Business Partners
7.4.7 Eurofins Scientific
7.4.7.1 Business Description & Financial Analysis
7.4.7.2 SWOT Analysis
7.4.7.3 Products & Services Offered
7.4.7.4 Strategic Alliances between Business Partners
7.4.8 UL Solutions
7.4.8.1 Business Description & Financial Analysis
7.4.8.2 SWOT Analysis
7.4.8.3 Products & Services Offered
7.4.8.4 Strategic Alliances between Business Partners
7.4.9 SteriPack Contract Manufacturing
7.4.9.1 Business Description & Financial Analysis
7.4.9.2 SWOT Analysis
7.4.9.3 Products & Services Offered
7.4.9.4 Strategic Alliances between Business Partners
7.4.10 DDL, Inc.
7.4.10.1 Business Description & Financial Analysis
7.4.10.2 SWOT Analysis
7.4.10.3 Products & Services Offered
7.4.10.4 Strategic Alliances between Business Partners
7.4.11 Other Companies
7.4.11.1 Business Description & Financial Analysis
7.4.11.2 SWOT Analysis
7.4.11.3 Products & Services Offered
7.4.11.4 Strategic Alliances between Business Partners
8 RESEARCH METHODOLOGY
8.1 Market Introduction
8.1.1 Market Definition
8.1.2 Market Scope & Segmentation
8.2 Information Procurement
8.2.1 Secondary Research
8.2.1.1 Purchased Databases
8.2.1.2 GMEs Internal Data Repository
8.2.1.3 Secondary Resources & Third Party Perspectives
8.2.1.4 Company Information Sources
8.2.2 Primary Research
8.2.2.1 Various Types of Respondents for Primary Interviews
8.2.2.2 Number of Interviews Conducted throughout the Research Process
8.2.2.3 Primary Stakeholders
8.2.2.4 Discussion Guide for Primary Participants
8.2.3 Expert Panels
8.2.3.1 Expert Panels Across 30+ Industry
8.2.4 Paid Local Experts
8.2.4.1 Paid Local Experts Across 30+ Industry Across each Region
8.3 Market Estimation
8.3.1 Top-Down Approach
8.3.1.1 Macro-Economic Indicators Considered
8.3.1.2 Micro-Economic Indicators Considered
8.3.2 Bottom Up Approach
8.3.2.1 Company Share Analysis Approach
8.3.2.2 Estimation of Potential Product Sales
8.4 Data Triangulation
8.4.1 Data Collection
8.4.2 Time Series, Cross Sectional & Panel Data Analysis
8.4.3 Cluster Analysis
8.5 Analysis and Output
8.5.1 Inhouse AI Based Real Time Analytics Tool
8.5.2 Output From Desk & Primary Research
8.6 Research Assumptions & Limitations
8.6.1 Research Assumptions
8.6.2 Research Limitations
LIST OF TABLES
1 Global Medical Device Package Validation Market, By Testing Type, 2021-2029 (USD Million)
2 Competitive Analysis Market, By Region, 2021-2029 (USD Million)
3 Physical Testing Market, By Region, 2021-2029 (USD Million)
4 Microbial Testing Market, By Region, 2021-2029 (USD Million)
5 visual Testing Market, By Region, 2021-2029 (USD Million)
6 chemical Testing Market, By Region, 2021-2029 (USD Million)
7 Global Medical Device Package Validation Market, By Device Class, 2021-2029 (USD Million)
8 Class I Devices Market, By Region, 2021-2029 (USD Million)
9 Class II Devices Market, By Region, 2021-2029 (USD Million)
10 Class III Devices Market, By Region, 2021-2029 (USD Million)
11 Regional Analysis, 2021-2029 (USD Million)
12 North America Medical Device Package Validation Market, By Testing Type, 2021-2029 (USD Million)
13 North America Medical Device Package Validation Market, By Device Class, 2021-2029 (USD Million)
14 North America Medical Device Package Validation Market, By COUNTRY, 2021-2029 (USD Million)
15 U.S. Medical Device Package Validation Market, By Testing Type, 2021-2029 (USD Million)
16 U.S. Medical Device Package Validation Market, By Device Class, 2021-2029 (USD Million)
17 Canada Medical Device Package Validation Market, By Testing Type, 2021-2029 (USD Million)
18 Canada Medical Device Package Validation Market, By Device Class, 2021-2029 (USD Million)
19 Mexico Medical Device Package Validation Market, By Testing Type, 2021-2029 (USD Million)
20 Mexico Medical Device Package Validation Market, By Device Class, 2021-2029 (USD Million)
21 Europe Medical Device Package Validation Market, By Testing Type, 2021-2029 (USD Million)
22 Europe Medical Device Package Validation Market, By Device Class, 2021-2029 (USD Million)
23 Europe Medical Device Package Validation Market, By Country, 2021-2029 (USD Million)
24 Germany Medical Device Package Validation Market, By Testing Type, 2021-2029 (USD Million)
25 Germany Medical Device Package Validation Market, By Device Class, 2021-2029 (USD Million)
26 U.K. Medical Device Package Validation Market, By Testing Type, 2021-2029 (USD Million)
27 U.K. Medical Device Package Validation Market, By Device Class, 2021-2029 (USD Million)
28 France Medical Device Package Validation Market, By Testing Type, 2021-2029 (USD Million)
29 France Medical Device Package Validation Market, By Device Class, 2021-2029 (USD Million)
30 Italy Medical Device Package Validation Market, By Testing Type, 2021-2029 (USD Million)
31 Italy Medical Device Package Validation Market, By Device Class, 2021-2029 (USD Million)
32 Spain Medical Device Package Validation Market, By Testing Type, 2021-2029 (USD Million)
33 Spain Medical Device Package Validation Market, By Device Class, 2021-2029 (USD Million)
34 Netherlands Medical Device Package Validation Market, By Testing Type, 2021-2029 (USD Million)
35 Netherlands Medical Device Package Validation Market, By Device Class, 2021-2029 (USD Million)
36 Rest Of Europe Medical Device Package Validation Market, By Testing Type, 2021-2029 (USD Million)
37 Rest Of Europe Medical Device Package Validation Market, By Device Class, 2021-2029 (USD Million)
38 Asia Pacific Medical Device Package Validation Market, By Testing Type, 2021-2029 (USD Million)
39 Asia Pacific Medical Device Package Validation Market, By Device Class, 2021-2029 (USD Million)
40 Asia Pacific Medical Device Package Validation Market, By Country, 2021-2029 (USD Million)
41 China Medical Device Package Validation Market, By Testing Type, 2021-2029 (USD Million)
42 China Medical Device Package Validation Market, By Device Class, 2021-2029 (USD Million)
43 Japan Medical Device Package Validation Market, By Testing Type, 2021-2029 (USD Million)
44 Japan Medical Device Package Validation Market, By Device Class, 2021-2029 (USD Million)
45 India Medical Device Package Validation Market, By Testing Type, 2021-2029 (USD Million)
46 India Medical Device Package Validation Market, By Device Class, 2021-2029 (USD Million)
47 South Korea Medical Device Package Validation Market, By Testing Type, 2021-2029 (USD Million)
48 South Korea Medical Device Package Validation Market, By Device Class, 2021-2029 (USD Million)
49 Singapore Medical Device Package Validation Market, By Testing Type, 2021-2029 (USD Million)
50 Singapore Medical Device Package Validation Market, By Device Class, 2021-2029 (USD Million)
51 Thailand Medical Device Package Validation Market, By Testing Type, 2021-2029 (USD Million)
52 Thailand Medical Device Package Validation Market, By Device Class, 2021-2029 (USD Million)
53 Malaysia Medical Device Package Validation Market, By Testing Type, 2021-2029 (USD Million)
54 Malaysia Medical Device Package Validation Market, By Device Class, 2021-2029 (USD Million)
55 Indonesia Medical Device Package Validation Market, By Testing Type, 2021-2029 (USD Million)
56 Indonesia Medical Device Package Validation Market, By Device Class, 2021-2029 (USD Million)
57 Vietnam Medical Device Package Validation Market, By Testing Type, 2021-2029 (USD Million)
58 Vietnam Medical Device Package Validation Market, By Device Class, 2021-2029 (USD Million)
59 Taiwan Medical Device Package Validation Market, By Testing Type, 2021-2029 (USD Million)
60 Taiwan Medical Device Package Validation Market, By Device Class, 2021-2029 (USD Million)
61 Rest of APAC Medical Device Package Validation Market, By Testing Type, 2021-2029 (USD Million)
62 Rest of APAC Medical Device Package Validation Market, By Device Class, 2021-2029 (USD Million)
63 Middle East and Africa Medical Device Package Validation Market, By Testing Type, 2021-2029 (USD Million)
64 Middle East and Africa Medical Device Package Validation Market, By Device Class, 2021-2029 (USD Million)
65 Middle East and Africa Medical Device Package Validation Market, By Country, 2021-2029 (USD Million)
66 Saudi Arabia Medical Device Package Validation Market, By Testing Type, 2021-2029 (USD Million)
67 Saudi Arabia Medical Device Package Validation Market, By Device Class, 2021-2029 (USD Million)
68 UAE Medical Device Package Validation Market, By Testing Type, 2021-2029 (USD Million)
69 UAE Medical Device Package Validation Market, By Device Class, 2021-2029 (USD Million)
70 Israel Medical Device Package Validation Market, By Testing Type, 2021-2029 (USD Million)
71 Israel Medical Device Package Validation Market, By Device Class, 2021-2029 (USD Million)
72 South Africa Medical Device Package Validation Market, By Testing Type, 2021-2029 (USD Million)
73 South Africa Medical Device Package Validation Market, By Device Class, 2021-2029 (USD Million)
74 Rest Of Middle East and Africa Medical Device Package Validation Market, By Testing Type, 2021-2029 (USD Million)
75 Rest Of Middle East and Africa Medical Device Package Validation Market, By Device Class, 2021-2029 (USD Million)
76 Central and South America Medical Device Package Validation Market, By Testing Type, 2021-2029 (USD Million)
77 Central and South America Medical Device Package Validation Market, By Device Class, 2021-2029 (USD Million)
78 Central and South America Medical Device Package Validation Market, By Country, 2021-2029 (USD Million)
79 Brazil Medical Device Package Validation Market, By Testing Type, 2021-2029 (USD Million)
80 Brazil Medical Device Package Validation Market, By Device Class, 2021-2029 (USD Million)
81 Chile Medical Device Package Validation Market, By Testing Type, 2021-2029 (USD Million)
82 Chile Medical Device Package Validation Market, By Device Class, 2021-2029 (USD Million)
83 Argentina Medical Device Package Validation Market, By Testing Type, 2021-2029 (USD Million)
84 Argentina Medical Device Package Validation Market, By Device Class, 2021-2029 (USD Million)
85 Rest Of Central and South America Medical Device Package Validation Market, By Testing Type, 2021-2029 (USD Million)
86 Rest Of Central and South America Medical Device Package Validation Market, By Device Class, 2021-2029 (USD Million)
87 Wetspak: Products & Services Offering
88 Life Science Outsourcing, Inc.: Products & Services Offering
89 Pro-Tech Design & Manufacturing: Products & Services Offering
90 WuXi AppTec Medical Device Testing: Products & Services Offering
91 Nelson Labs: Products & Services Offering
92 KEYSTONE PACKAGE TESTING: Products & Services Offering
93 Eurofins Scientific : Products & Services Offering
94 UL Solutions: Products & Services Offering
95 SteriPack Contract Manufacturing, Inc: Products & Services Offering
96 DDL, Inc.: Products & Services Offering
97 Other Companies: Products & Services Offering
LIST OF FIGURES
1 Global Medical Device Package Validation Market Overview
2 Global Medical Device Package Validation Market Value From 2021-2029 (USD Million)
3 Global Medical Device Package Validation Market Share, By Testing Type (2023)
4 Global Medical Device Package Validation Market Share, By Device Class (2023)
5 Global Medical Device Package Validation Market, By Region (Asia Pacific Market)
6 Technological Trends In Global Medical Device Package Validation Market
7 Four Quadrant Competitor Positioning Matrix
8 Impact Of Macro & Micro Indicators On The Market
9 Impact Of Key Drivers On The Global Medical Device Package Validation Market
10 Impact Of Challenges On The Global Medical Device Package Validation Market
11 Porter’s Five Forces Analysis
12 Global Medical Device Package Validation Market: By Testing Type Scope Key Takeaways
13 Global Medical Device Package Validation Market, By Testing Type Segment: Revenue Growth Analysis
14 Competitive Analysis Market, By Region, 2021-2029 (USD Million)
15 Physical Testing Market, By Region, 2021-2029 (USD Million)
16 Microbial Testing Market, By Region, 2021-2029 (USD Million)
17 Chemical Testing Market, By Region, 2021-2029 (USD Million)
18 Visual Testing Market, By Region, 2021-2029 (USD Million)
19 Global Medical Device Package Validation Market: By Device Class Scope Key Takeaways
20 Global Medical Device Package Validation Market, By Device Class Segment: Revenue Growth Analysis
21 Class I Devices Market, By Region, 2021-2029 (USD Million)
22 Class II Devices Market, By Region, 2021-2029 (USD Million)
23 Class III Devices Market, By Region, 2021-2029 (USD Million)
24 Regional Segment: Revenue Growth Analysis
25 Global Medical Device Package Validation Market: Regional Analysis
26 North America Medical Device Package Validation Market Overview
27 North America Medical Device Package Validation Market, By Testing Type
28 North America Medical Device Package Validation Market, By Device Class
29 North America Medical Device Package Validation Market, By Country
30 U.S. Medical Device Package Validation Market, By Testing Type
31 U.S. Medical Device Package Validation Market, By Device Class
32 Canada Medical Device Package Validation Market, By Testing Type
33 Canada Medical Device Package Validation Market, By Device Class
34 Mexico Medical Device Package Validation Market, By Testing Type
35 Mexico Medical Device Package Validation Market, By Device Class
36 Four Quadrant Positioning Matrix
37 Company Market Share Analysis
38 Wetspak: Company Snapshot
39 Wetspak: SWOT Analysis
40 Wetspak: Geographic Presence
41 Life Science Outsourcing, Inc.: Company Snapshot
42 Life Science Outsourcing, Inc.: SWOT Analysis
43 Life Science Outsourcing, Inc.: Geographic Presence
44 Pro-Tech Design & Manufacturing: Company Snapshot
45 Pro-Tech Design & Manufacturing: SWOT Analysis
46 Pro-Tech Design & Manufacturing: Geographic Presence
47 WuXi AppTec Medical Device Testing: Company Snapshot
48 WuXi AppTec Medical Device Testing: Swot Analysis
49 WuXi AppTec Medical Device Testing: Geographic Presence
50 Nelson Labs: Company Snapshot
51 Nelson Labs: SWOT Analysis
52 Nelson Labs: Geographic Presence
53 Keystone Package Testing: Company Snapshot
54 Keystone Package Testing: SWOT Analysis
55 Keystone Package Testing: Geographic Presence
56 Eurofins Scientific : Company Snapshot
57 Eurofins Scientific : SWOT Analysis
58 Eurofins Scientific : Geographic Presence
59 UL Solutions: Company Snapshot
60 UL Solutions: SWOT Analysis
61 UL Solutions: Geographic Presence
62 SteriPack Contract Manufacturing, Inc.: Company Snapshot
63 SteriPack Contract Manufacturing, Inc.: SWOT Analysis
64 SteriPack Contract Manufacturing, Inc.: Geographic Presence
65 DDL, Inc.: Company Snapshot
66 DDL, Inc.: SWOT Analysis
67 DDL, Inc.: Geographic Presence
68 Other Companies: Company Snapshot
69 Other Companies: SWOT Analysis
70 Other Companies: Geographic Presence
The Global Medical Device Package Validation Market has been studied from the year 2019 till 2029. However, the CAGR provided in the report is from the year 2024 to 2029. The research methodology involved three stages: Desk research, Primary research, and Analysis & Output from the entire research process.

The desk research involved a robust background study which meant referring to paid and unpaid databases to understand the market dynamics; mapping contracts from press releases; identifying the key players in the market, studying their product portfolio, competition level, annual reports/SEC filings & investor presentations; and learning the demand and supply-side analysis for the Medical Device Package Validation Market.

The primary research activity included telephonic conversations with more than 50 tier 1 industry consultants, distributors, and end-use product manufacturers.

Finally, based on the above thorough research process, an in-depth analysis was carried out considering the following aspects: market attractiveness, current & future market trends, market share analysis, SWOT analysis of the company and customer analytics.

Frequently Asked Questions
Tailor made solutions just for you
80% of our clients seek made-to-order reports. How do you want us to tailor yours?
OUR CLIENTS