
Global Omics-Based Clinical Trials Market Size, Trends & Analysis - Forecasts to 2028 By Phase Type (Phase I, Phase II, Phase III, and Phase IV), By Study Design Type (Interventional Studies, Observational Studies, and Expanded Access Studies), By Indication Type (Oncology, Cardiology, Respiratory Diseases, Skin Diseases, CNS Diseases, Immunology, Genetic Diseases, and Others), and By Region (North America, Asia Pacific, Central & South America, Europe, and Middle East and Africa), Competitive Landscape, Company Market Share Analysis, and End User Analysis
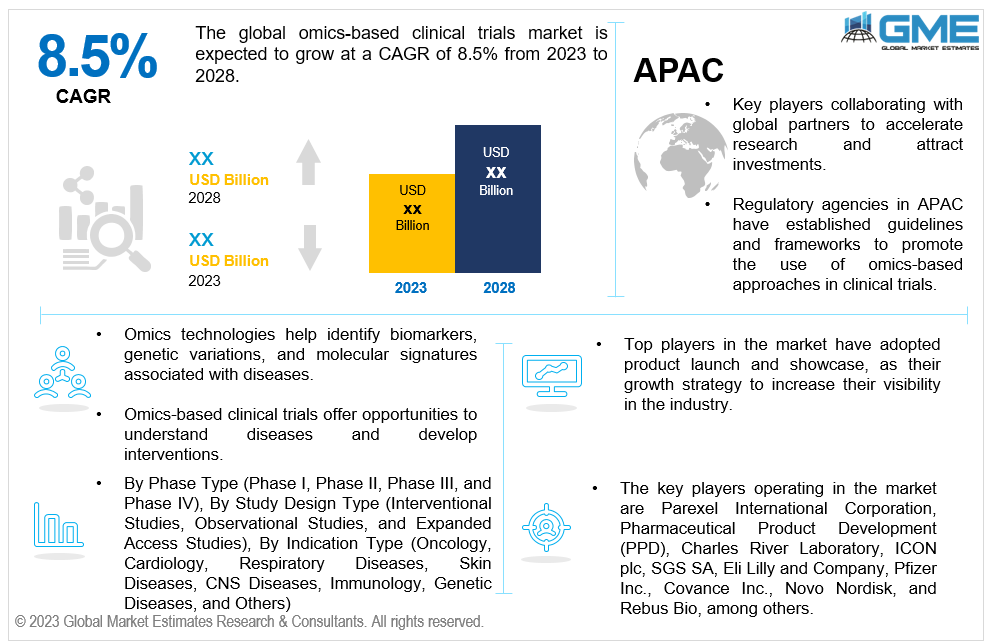
The global omics-based clinical trials market is expected to grow at a CAGR of 8.5% from 2023 to 2028. The omics-based clinical trials market is the sector that focuses on conducting clinical trials using omics technologies. Omics refers to the comprehensive study of various biological components, such as genomics, transcriptomics, proteomics, metabolomics, and epigenomics, which enable researchers to analyse and understand the molecular profiles and interactions within an organism, leading to advancements in personalized medicine and targeted therapies. The omics-based clinical trials market includes various stakeholders, such as pharmaceutical and biotechnology companies, academic research institutions, contract research organizations (CROs), and government agencies, who collaborate to design and conduct clinical trials that incorporate omics technologies, aiming to accelerate the development of targeted therapies and improve patient outcomes. With ongoing advancements and increasing adoption, omics-based trials are expected to play a pivotal role in shaping the future of precision medicine and personalized healthcare.
The omics-based clinical trials market growth is driven by several market drivers, such as technological advancements, precision medicine, the increasing burden of chronic diseases, regulatory support, collaborations and partnerships, cost reduction and accessibility, and growing data availability and analysis capabilities. Technology has improved the efficiency, accuracy, and cost-effectiveness of analysing biological data, while precision medicine aims to provide targeted treatments based on an individual's genetic and molecular characteristics. Regulatory agencies have recognized the value of omics technologies in drug development and patient care, and collaborations and partnerships among stakeholders foster knowledge sharing, accelerate research, and drive the market growth. Cost reduction and accessibility has enabled wider adoption of omics-based approaches, leading to an expansion of the market. Data availability and analysis capabilities have enabled researchers to extract meaningful insights from complex biological data, driving the demand for omics-based clinical trials.
The omics-based clinical trials market presents numerous opportunities, but there are several restraints that can impact its growth and adoption. These include high costs, data management and analysis challenges, standardization and reproducibility, ethical and legal considerations, and privacy and data security concerns. Additionally, the need for skilled personnel and infrastructure can be a significant restraint. Omics-based trials must address patient confidentiality, informed consent, limited clinical validation, integration into clinical practice, and regulatory and reimbursement challenges. Robust clinical validation studies are necessary to bridge the gap between research findings and clinical applications. Integrating omics-based approaches into routine clinical practice requires changes in workflows, data management systems, and clinical decision-making processes.
Based on phase type, the market is segmented into Phase I, Phase II, Phase III, and Phase IV. Phase II is expected to be the largest segment during the forecast period. Phase 2 trials are essential for evaluating the safety, efficacy, and optimal dosage of a new intervention or treatment approach, mitigating risks, supporting regulatory agencies, and influencing investment decisions.
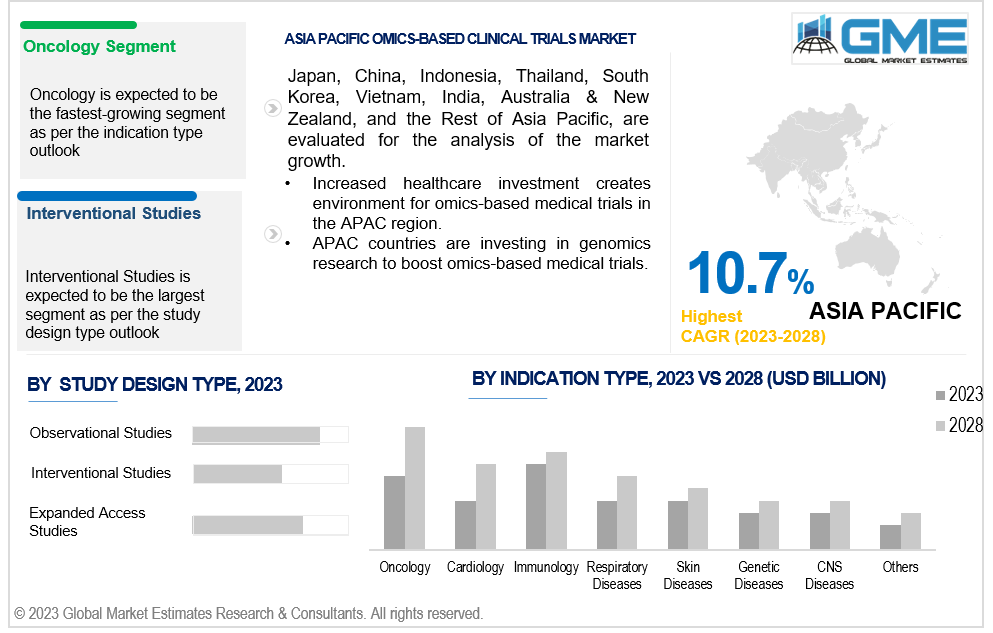
Based on study design type, the market is segmented into interventional studies, observational studies, and expanded access studies. Interventional studies is expected to be the largest segment during the forecast period. Interventional studies are essential for the development and evaluation of new therapeutic interventions, providing data to support regulatory submissions, validating clinical utility, and attracting investor interest.
Based on indication type, the market is segmented into oncology, cardiology, respiratory diseases, skin diseases, CNS diseases, immunology, genetic diseases, and others. Oncology is expected to be the largest segment during the forecast period. Cancer is a leading cause of death worldwide, leading to extensive research and clinical trials utilizing omics technologies. Omics technologies provide insights into genetic mutations, gene expression patterns, and protein profiles, leading to personalized medicine opportunities, regulatory approvals, industry investment, and patient advocacy.
North America is analysed to be the largest region in the global omics-based clinical trials market during the forecast period. North America has a well-established and advanced healthcare infrastructure, including robust research institutions, leading academic centers, and top-tier medical facilities. It has been at the forefront of technological advancements in the field of omics, and has access to significant research funding and support from government agencies, private organizations, and venture capitalists. It is home to several major pharmaceutical and biotechnology companies that actively invest in omics-based research and development, and has provided guidelines and frameworks for the integration of omics technologies in clinical trials. These regulatory initiatives support the safe and effective use of omics-based approaches, providing clarity and facilitating the adoption of these technologies.
Asia Pacific is expected to be the fastest-growing region across the global omics-based clinical trials market with a CAGR of over 10.7%, owing to the high presence of patient population, increasing investments in research and development, rising healthcare expenditure, and a favorable regulatory environment for omics-based clinical trials. This has created a conducive environment for the adoption of omics technologies, leading to greater support and funding for omics-based research and clinical trials. The APAC region has established guidelines and frameworks to promote the use of omics-based approaches, and is collaborating with international partners to leverage expertise, share resources, and conduct multi-center trials. Precision medicine, which tailors medical treatments to individual patients based on their genetic and molecular profiles, has gained attention in the region, leading to a rise in omics-based clinical trials.
The key players operating in the market are Parexel International Corporation, Pharmaceutical Product Development (PPD), Charles River Laboratory, ICON plc, SGS SA, Eli Lilly and Company, Pfizer Inc., Covance Inc., Novo Nordisk, and Rebus Bio, among others. The global omics-based clinical trials market has observed several strategic alliances between companies to launch new products with added functionalities and maintain revenue share & profitability. Organic and inorganic growth strategies adopted by small players have been the highlight of this market.
Please note: This is not an exhaustive list of companies profiled in the report.
1 STRATEGIC INSIGHTS ON NEW REVENUE POCKETS
1.1 Strategic Opportunity & Attractiveness Analysis
1.1.1 Hot Revenue Pockets
1.1.2 Market Attractiveness Score
1.1.3 Revenue Impacting Opportunity
1.1.4 High Growing Region/Country
1.1.5 Competitor Analysis
1.1.6 Consumer Analysis
1.2 Global Market Estimates' View
1.3 Strategic Insights across Business Functions
1.3.1 For Chief Executive Officers
1.3.2 For Chief Marketing Officers
1.3.3 For Chief Strategy Officers
1.4 Evaluate the Potential of your Existing Business Lines vs. New Lines to Enter Into
2 TECHNOLOGICAL TRENDS
2.1 Technological Adoption Rate
2.2 Current Trend Impact Analysis
2.3 Future Trend Impact Analysis
2.4 Data Metrics on Feed Stocks
3 GLOBAL MARKET OUTLOOK
3.1 Market Pyramid Analysis
3.1.1 Introduction
3.1.2 Adjacent Market Opportunities
3.1.3 Ancillary Market Opportunities
3.2 Demand Side Analysis
3.2.1 Market Drivers: Impact Analysis
3.2.2 Market Restraints: Impact Analysis
3.2.3 Market Opportunities: Impact Analysis
3.2.4 Market Challenges: Impact Analysis
3.3 Supply Side Analysis
3.3.1 Porter’s Five Forces Analysis
3.3.1.1 Threat of New Entrants
3.3.1.2 Threat of New Substitutes
3.3.1.3 Bargaining Power of Suppliers
3.3.1.4 Bargaining Power of Buyers
3.3.1.5 Intensity of Competitive Rivalry
3.3.2 SWOT Analysis; By Factor (Political & Legal, Economic, and Technological)
3.3.2.1 Political Landscape
3.3.2.2 Economic Landscape
3.3.2.3 Social Landscape
3.3.2.4 Technology Landscape
3.3.3 Value Chain Analysis
3.3.4 Trend Analysis
3.3.5 Gap Analysis
3.3.6 Cost Analysis
4 GLOBAL OMICS-BASED CLINICAL TRIALS MARKET, BY STUDY DESIGN TYPE
4.1 Introduction
4.2 Omics-Based Clinical Trials Market: Study Design Type Scope Key Takeaways
4.3 Revenue Growth Analysis, 2022 & 2028
4.4 Interventional Studies
4.4.1 Interventional Studies Market Estimates and Forecast, 2020-2028 (USD Million)
4.5 Observational Studies
4.5.1 Observational Studies Market Estimates and Forecast, 2020-2028 (USD Million)
4.6 Expanded Access Studies
4.6.1 Expanded Access Studies Market Estimates and Forecast, 2020-2028 (USD Million)
5 GLOBAL OMICS-BASED CLINICAL TRIALS MARKET, BY PHASE TYPE
5.1 Introduction
5.2 Omics-Based Clinical Trials Market: Phase Type Scope Key Takeaways
5.3 Revenue Growth Analysis, 2022 & 2028
5.4 Phase I
5.4.1 Phase I Market Estimates and Forecast, 2020-2028 (USD Million)
5.5 Phase II
5.5.1 Phase II Market Estimates and Forecast, 2020-2028 (USD Million)
5.6 Phase III
5.6.1 Phase III Market Estimates and Forecast, 2020-2028 (USD Million)
5.7 Phase IV
5.7.1 Phase IV Market Estimates and Forecast, 2020-2028 (USD Million)
6 GLOBAL OMICS-BASED CLINICAL TRIALS MARKET, BY INDICATION TYPE
6.1 Introduction
6.2 Omics-Based Clinical Trials Market: Indication Type Scope Key Takeaways
6.3 Revenue Growth Analysis, 2022 & 2028
6.4 Oncology
6.4.1 Oncology Market Estimates and Forecast, 2020-2028 (USD Million)
6.5 Cardiology
6.5.1 Cardiology Market Estimates and Forecast, 2020-2028 (USD Million)
6.6 Respiratory Diseases
6.9.1 Respiratory Diseases Market Estimates and Forecast, 2020-2028 (USD Million)
6.7 CNS Diseases
6.7.1 CNS Diseases Market Estimates and Forecast, 2020-2028 (USD Million)
6.8 Genetic Diseases
6.8.1 Genetic Diseases Market Estimates and Forecast, 2020-2028 (USD Million)
6.9 Skin Diseases
6.9.1 Skin Diseases Market Estimates and Forecast, 2020-2028 (USD Million)
6.10 Other Indications
6.10.1 Other Indications Market Estimates and Forecast, 2020-2028 (USD Million)
7 GLOBAL OMICS-BASED CLINICAL TRIALS MARKET, BY REGION
7.1 Introduction
7.2 North America Omics-Based Clinical Trials Market Estimates and Forecast, 2020-2028 (USD Million)
7.2.1 By Study Design Type
7.2.2 By Phase Type
7.2.3 By Indication Type
7.2.4 By Country
7.2.4.1 U.S. Omics-Based Clinical Trials Market Estimates and Forecast, 2020-2028 (USD Million)
7.2.4.1.1 By Study Design Type
7.2.4.1.2 By Phase Type
7.2.4.1.3 By Indication Type
7.2.4.2 Canada Omics-Based Clinical Trials Market Estimates and Forecast, 2020-2028 (USD Million)
7.2.4.2.1 By Study Design Type
7.2.4.2.2 By Phase Type
7.2.4.2.3 By Indication Type
7.2.4.3 Mexico Omics-Based Clinical Trials Market Estimates and Forecast, 2020-2028 (USD Million)
7.2.4.3.1 By Study Design Type
7.2.4.3.2 By Phase Type
7.2.4.3.3 By Indication Type
7.3 Europe Omics-Based Clinical Trials Market Estimates and Forecast, 2020-2028 (USD Million)
7.3.1 By Study Design Type
7.3.2 By Phase Type
7.3.3 By Indication Type
7.3.4 By Country
7.3.4.1 Germany Omics-Based Clinical Trials Market Estimates and Forecast, 2020-2028 (USD Million)
7.3.4.1.1 By Study Design Type
7.3.4.1.2 By Phase Type
7.3.4.1.3 By Indication Type
7.3.4.2 U.K. Omics-Based Clinical Trials Market Estimates and Forecast, 2020-2028 (USD Million)
7.3.4.2.1 By Study Design Type
7.3.4.2.2 By Phase Type
7.3.4.2.3 By Indication Type
7.3.4.3 France Omics-Based Clinical Trials Market Estimates and Forecast, 2020-2028 (USD Million)
7.3.4.3.1 By Study Design Type
7.3.4.3.2 By Phase Type
7.3.4.3.3 By Indication Type
7.3.4.4 Italy Omics-Based Clinical Trials Market Estimates and Forecast, 2020-2028 (USD Million)
7.3.4.4.1 By Study Design Type
7.3.4.4.2 By Phase Type
7.2.4.4.3 By Indication Type
7.3.4.5 Spain Omics-Based Clinical Trials Market Estimates and Forecast, 2020-2028 (USD Million)
7.3.4.5.1 By Study Design Type
7.3.4.5.2 By Phase Type
7.2.4.5.3 By Indication Type
7.3.4.6 Netherlands Omics-Based Clinical Trials Market Estimates and Forecast, 2020-2028 (USD Million)
7.3.4.7.1 By Study Design Type
7.3.4.7.2 By Phase Type
7.2.4.7.3 By Indication Type
7.3.4.7 Rest of Europe Omics-Based Clinical Trials Market Estimates and Forecast, 2020-2028 (USD Million)
7.3.4.7.1 By Study Design Type
7.3.4.7.2 By Phase Type
7.2.4.7.3 By Indication Type
7.4 Asia Pacific Omics-Based Clinical Trials Market Estimates and Forecast, 2020-2028 (USD Million)
7.4.1 By Study Design Type
7.4.2 By Phase Type
7.4.3 By Indication Type
7.4.4 By Country
7.4.4.1 China Omics-Based Clinical Trials Market Estimates and Forecast, 2020-2028 (USD Million)
7.4.4.1.1 By Study Design Type
7.4.4.1.2 By Phase Type
7.4.4.1.3 By Indication Type
7.4.4.2 Japan Omics-Based Clinical Trials Market Estimates and Forecast, 2020-2028 (USD Million)
7.4.4.2.1 By Study Design Type
7.4.4.2.2 By Phase Type
7.4.4.2.3 By Indication Type
7.4.4.3 India Omics-Based Clinical Trials Market Estimates and Forecast, 2020-2028 (USD Million)
7.4.4.3.1 By Study Design Type
7.4.4.3.2 By Phase Type
7.4.4.3.3 By Indication Type
7.4.4.4 South Korea Omics-Based Clinical Trials Market Estimates and Forecast, 2020-2028 (USD Million)
7.4.4.4.1 By Study Design Type
7.4.4.4.2 By Phase Type
7.4.4.4.3 By Indication Type
7.4.4.5 Singapore Omics-Based Clinical Trials Market Estimates and Forecast, 2020-2028 (USD Million)
7.4.4.5.1 By Study Design Type
7.4.4.5.2 By Phase Type
7.4.4.5.3 By Indication Type
7.4.4.6 Malaysia Omics-Based Clinical Trials Market Estimates and Forecast, 2020-2028 (USD Million)
7.4.4.7.1 By Study Design Type
7.4.4.7.2 By Phase Type
7.4.4.7.3 By Indication Type
7.4.4.7 Thailand Omics-Based Clinical Trials Market Estimates and Forecast, 2020-2028 (USD Million)
7.4.4.7.1 By Study Design Type
7.4.4.7.2 By Phase Type
7.4.4.7.3 By Indication Type
7.4.4.8 Indonesia Omics-Based Clinical Trials Market Estimates and Forecast, 2020-2028 (USD Million)
7.4.4.8.1 By Study Design Type
7.4.4.8.2 By Phase Type
7.4.4.8.3 By Indication Type
7.4.4.9 Vietnam Omics-Based Clinical Trials Market Estimates and Forecast, 2020-2028 (USD Million)
7.4.4.9.1 By Study Design Type
7.4.4.9.2 By Phase Type
7.4.4.9.3 By Indication Type
7.4.4.10 Taiwan Omics-Based Clinical Trials Market Estimates and Forecast, 2020-2028 (USD Million)
7.4.4.10.1 By Study Design Type
7.4.4.10.2 By Phase Type
7.4.4.10.3 By Indication Type
7.4.4.11 Rest of Asia Pacific Omics-Based Clinical Trials Market Estimates and Forecast, 2020-2028 (USD Million)
7.4.4.11.1 By Study Design Type
7.4.4.11.2 By Phase Type
7.4.4.11.3 By Indication Type
7.5 Middle East and Africa Omics-Based Clinical Trials Market Estimates and Forecast, 2020-2028 (USD Million)
7.5.1 By Study Design Type
7.5.2 By Phase Type
7.5.3 By Indication Type
7.5.4 By Country
7.5.4.1 Saudi Arabia Omics-Based Clinical Trials Market Estimates and Forecast, 2020-2028 (USD Million)
7.5.4.1.1 By Study Design Type
7.5.4.1.2 By Phase Type
7.5.4.1.3 By Indication Type
7.5.4.2 U.A.E. Omics-Based Clinical Trials Market Estimates and Forecast, 2020-2028 (USD Million)
7.5.4.2.1 By Study Design Type
7.5.4.2.2 By Phase Type
7.5.4.2.3 By Indication Type
7.5.4.3 Israel Omics-Based Clinical Trials Market Estimates and Forecast, 2020-2028 (USD Million)
7.5.4.3.1 By Study Design Type
7.5.4.3.2 By Phase Type
7.5.4.3.3 By Indication Type
7.5.4.4 South Africa Omics-Based Clinical Trials Market Estimates and Forecast, 2020-2028 (USD Million)
7.5.4.4.1 By Study Design Type
7.5.4.4.2 By Phase Type
7.5.4.4.3 By Indication Type
7.5.4.5 Rest of Middle East and Africa Omics-Based Clinical Trials Market Estimates and Forecast, 2020-2028 (USD Million)
7.5.4.5.1 By Study Design Type
7.5.4.5.2 By Phase Type
7.5.4.5.2 By Indication Type
7.6 Central & South America Omics-Based Clinical Trials Market Estimates and Forecast, 2020-2028 (USD Million)
7.7.1 By Study Design Type
7.7.2 By Phase Type
7.7.3 By Indication Type
7.7.4 By Country
7.7.4.1 Brazil Omics-Based Clinical Trials Market Estimates and Forecast, 2020-2028 (USD Million)
7.7.4.1.1 By Study Design Type
7.7.4.1.2 By Phase Type
7.7.4.1.3 By Indication Type
7.7.4.2 Argentina Omics-Based Clinical Trials Market Estimates and Forecast, 2020-2028 (USD Million)
7.7.4.2.1 By Study Design Type
7.7.4.2.2 By Phase Type
7.7.4.2.3 By Indication Type
7.7.4.3 Chile Omics-Based Clinical Trials Market Estimates and Forecast, 2020-2028 (USD Million)
7.7.4.3.1 By Study Design Type
7.7.4.3.2 By Phase Type
7.7.4.3.3 By Indication Type
7.7.4.4 Rest of Central & South America Omics-Based Clinical Trials Market Estimates and Forecast, 2020-2028 (USD Million)
7.7.4.4.1 By Study Design Type
7.7.4.4.2 By Phase Type
7.7.4.4.3 By Indication Type
8 COMPETITIVE LANDCAPE
8.1 Company Market Share Analysis
8.2 Four Quadrant Positioning Matrix
8.2.1 Market Leaders
8.2.2 Market Visionaries
8.2.3 Market Challengers
8.2.4 Niche Market Players
8.3 Vendor Landscape
8.3.1 North America
8.3.2 Europe
8.3.3 Asia Pacific
8.3.4 Rest of the World
8.4 Company Profiles
8.4.1 Pfizer Inc.
8.4.1.1 Business Description & Financial Analysis
8.4.1.2 SWOT Analysis
8.4.1.3 Products & Services Offered
8.4.1.4 Strategic Alliances between Business Partners
8.4.2 Covance Inc.
8.4.2.1 Business Description & Financial Analysis
8.4.2.2 SWOT Analysis
8.4.2.3 Products & Services Offered
8.4.2.4 Strategic Alliances between Business Partners
8.4.3 Parexel International Corporation
8.4.3.1 Business Description & Financial Analysis
8.4.3.2 SWOT Analysis
8.4.3.3 Products & Services Offered
8.4.3.4 Strategic Alliances between Business Partners
8.4.4 Charles River Laboratory
8.4.4.1 Business Description & Financial Analysis
8.4.4.2 SWOT Analysis
8.4.4.3 Products & Services Offered
8.4.4.4 Strategic Alliances between Business Partners
8.4.5 Icon Plc
8.4.5.1 Business Description & Financial Analysis
8.4.5.2 SWOT Analysis
8.4.5.3 Products & Services Offered
8.4.5.4 Strategic Alliances between Business Partners
8.4.6 Eli Lilly and Company
8.4.7.1 Business Description & Financial Analysis
8.4.7.2 SWOT Analysis
8.4.7.3 Products & Services Offered
8.4.7.4 Strategic Alliances between Business Partners
8.4.7 Rebus Bio
8.4.7.1 Business Description & Financial Analysis
8.4.7.2 SWOT Analysis
8.4.7.3 Products & Services Offered
8.4.8.4 Strategic Alliances between Business Partners
8.4.8 Novo Nordisk
8.4.8.1 Business Description & Financial Analysis
8.4.8.2 SWOT Analysis
8.4.8.3 Products & Services Offered
8.4.8.4 Strategic Alliances between Business Partners
8.4.9 Other Companies
8.4.10.1 Business Description & Financial Analysis
8.4.10.2 SWOT Analysis
8.4.10.3 Products & Services Offered
8.4.10.4 Strategic Alliances between Business Partners
9 RESEARCH METHODOLOGY
9.1 Market Introduction
9.1.1 Market Definition
9.1.2 Market Scope & Segmentation
9.2 Information Procurement
9.2.1 Secondary Research
9.2.1.1 Purchased Databases
9.2.1.2 GMEs Internal Data Repository
9.2.1.3 Secondary Resources & Third Party Perspectives
9.2.1.4 Company Information Sources
9.2.2 Primary Research
9.2.2.1 Various Types of Respondents for Primary Interviews
9.2.2.2 Number of Interviews Conducted throughout the Research Process
9.2.2.3 Primary Stakeholders
9.2.2.4 Discussion Guide for Primary Participants
9.2.3 Expert Panels
9.2.3.1 Expert Panels Across 30+ Industry
9.2.4 Paid Local Experts
9.2.4.1 Paid Local Experts Across 30+ Industry Across each Region
9.3 Market Estimation
9.3.1 Top-Down Approach
9.3.1.1 Macro-Economic Indicators Considered
9.3.1.2 Micro-Economic Indicators Considered
9.3.2 Bottom Up Approach
9.3.2.1 Company Share Analysis Approach
9.3.2.2 Estimation of Potential Product Sales
9.4 Data Triangulation
9.4.1 Data Collection
9.4.2 Time Series, Cross Sectional & Panel Data Analysis
9.4.3 Cluster Analysis
9.5 Analysis and Output
9.5.1 Inhouse AI Based Real Time Analytics Tool
9.5.2 Output From Desk & Primary Research
9.6 Research Assumptions & Limitations
9.7.1 Research Assumptions
9.7.2 Research Limitations
LIST OF TABLES
1 Global Omics-Based Clinical Trials Market, By Study Design Type, 2020-2028 (USD Mllion)
2 Interventional Studies Market, By Region, 2020-2028 (USD Mllion)
3 Observational Studies Market, By Region, 2020-2028 (USD Mllion)
4 Expanded Access Studies Market, By Region, 2020-2028 (USD Mllion)
5 Global Omics-Based Clinical Trials Market, By Phase Type, 2020-2028 (USD Mllion)
6 Phase I Market, By Region, 2020-2028 (USD Mllion)
7 Phase II Market, By Region, 2020-2028 (USD Mllion)
8 Phase III Market, By Region, 2020-2028 (USD Mllion)
9 Phase IV Market, By Region, 2020-2028 (USD Mllion)
10 Global Omics-Based Clinical Trials Market, By Indication Type, 2020-2028 (USD Mllion)
11 Oncology Market, By Region, 2020-2028 (USD Mllion)
12 Cardiology Market, By Region, 2020-2028 (USD Mllion)
13 Respiratory Diseases Market, By Region, 2020-2028 (USD Mllion)
14 CNS Diseases Market, By Region, 2020-2028 (USD Mllion)
15 Genetic Diseases Market, By Region, 2020-2028 (USD Mllion)
16 Skin Diseases Market, By Region, 2020-2028 (USD Mllion)
17 Other Indications Market, By Region, 2020-2028 (USD Mllion)
18 Regional Analysis, 2020-2028 (USD Mllion)
19 North America Omics-Based Clinical Trials Market, By Study Design Type, 2020-2028 (USD Million)
20 North America Omics-Based Clinical Trials Market, By Phase Type, 2020-2028 (USD Million)
21 North America Omics-Based Clinical Trials Market, By Indication Type, 2020-2028 (USD Million)
22 North America Omics-Based Clinical Trials Market, By Country, 2020-2028 (USD Million)
23 U.S Omics-Based Clinical Trials Market, By Study Design Type, 2020-2028 (USD Million)
24 U.S Omics-Based Clinical Trials Market, By Phase Type, 2020-2028 (USD Million)
25 U.S Omics-Based Clinical Trials Market, By Indication Type, 2020-2028 (USD Million)
26 Canada Omics-Based Clinical Trials Market, By Study Design Type, 2020-2028 (USD Million)
27 Canada Omics-Based Clinical Trials Market, By Phase Type, 2020-2028 (USD Million)
28 Canada Omics-Based Clinical Trials Market, By Indication Type, 2020-2028 (USD Million)
29 Mexico Omics-Based Clinical Trials Market, By Study Design Type, 2020-2028 (USD Million)
30 Mexico Omics-Based Clinical Trials Market, By Phase Type, 2020-2028 (USD Million)
31 Mexico Omics-Based Clinical Trials Market, By Indication Type, 2020-2028 (USD Million)
32 Europe Omics-Based Clinical Trials Market, By Study Design Type, 2020-2028 (USD Million)
33 Europe Omics-Based Clinical Trials Market, By Phase Type, 2020-2028 (USD Million)
34 Europe Omics-Based Clinical Trials Market, By Indication Type, 2020-2028 (USD Million)
35 Germany Omics-Based Clinical Trials Market, By Study Design Type, 2020-2028 (USD Million)
36 Germany Omics-Based Clinical Trials Market, By Phase Type, 2020-2028 (USD Million)
37 Germany Omics-Based Clinical Trials Market, By Indication Type, 2020-2028 (USD Million)
38 UK Omics-Based Clinical Trials Market, By Study Design Type, 2020-2028 (USD Million)
39 UK Omics-Based Clinical Trials Market, By Phase Type, 2020-2028 (USD Million)
40 UK Omics-Based Clinical Trials Market, By Indication Type, 2020-2028 (USD Million)
41 France Omics-Based Clinical Trials Market, By Study Design Type, 2020-2028 (USD Million)
42 France Omics-Based Clinical Trials Market, By Phase Type, 2020-2028 (USD Million)
43 France Omics-Based Clinical Trials Market, By Indication Type, 2020-2028 (USD Million)
44 Italy Omics-Based Clinical Trials Market, By Study Design Type, 2020-2028 (USD Million)
45 Italy Omics-Based Clinical Trials Market, By T Phase Type Type, 2020-2028 (USD Million)
46 Italy Omics-Based Clinical Trials Market, By Indication Type, 2020-2028 (USD Million)
47 Spain Omics-Based Clinical Trials Market, By Study Design Type, 2020-2028 (USD Million)
48 Spain Omics-Based Clinical Trials Market, By Phase Type, 2020-2028 (USD Million)
49 Spain Omics-Based Clinical Trials Market, By Indication Type, 2020-2028 (USD Million)
50 Rest Of Europe Omics-Based Clinical Trials Market, By Study Design Type, 2020-2028 (USD Million)
51 Rest Of Europe Omics-Based Clinical Trials Market, By Phase Type, 2020-2028 (USD Million)
52 Rest of Europe Omics-Based Clinical Trials Market, By Indication Type, 2020-2028 (USD Million)
53 Asia Pacific Omics-Based Clinical Trials Market, By Study Design Type, 2020-2028 (USD Million)
54 Asia Pacific Omics-Based Clinical Trials Market, By Phase Type, 2020-2028 (USD Million)
55 Asia Pacific Omics-Based Clinical Trials Market, By Indication Type, 2020-2028 (USD Million)
56 Asia Pacific Omics-Based Clinical Trials Market, By Country, 2020-2028 (USD Million)
57 China Omics-Based Clinical Trials Market, By Study Design Type, 2020-2028 (USD Million)
58 China Omics-Based Clinical Trials Market, By Phase Type, 2020-2028 (USD Million)
59 China Omics-Based Clinical Trials Market, By Indication Type, 2020-2028 (USD Million)
60 India Omics-Based Clinical Trials Market, By Study Design Type, 2020-2028 (USD Million)
61 India Omics-Based Clinical Trials Market, By Phase Type, 2020-2028 (USD Million)
62 India Omics-Based Clinical Trials Market, By Indication Type, 2020-2028 (USD Million)
63 Japan Omics-Based Clinical Trials Market, By Study Design Type, 2020-2028 (USD Million)
64 Japan Omics-Based Clinical Trials Market, By Phase Type, 2020-2028 (USD Million)
65 Japan Omics-Based Clinical Trials Market, By Indication Type, 2020-2028 (USD Million)
66 South Korea Omics-Based Clinical Trials Market, By Study Design Type, 2020-2028 (USD Million)
67 South Korea Omics-Based Clinical Trials Market, By Phase Type, 2020-2028 (USD Million)
68 South Korea Omics-Based Clinical Trials Market, By Indication Type, 2020-2028 (USD Million)
69 Middle East and Africa Omics-Based Clinical Trials Market, By Study Design Type, 2020-2028 (USD Million)
70 Middle East and Africa Omics-Based Clinical Trials Market, By Phase Type, 2020-2028 (USD Million)
71 Middle East and Africa Omics-Based Clinical Trials Market, By Indication Type, 2020-2028 (USD Million)
72 Middle East and Africa Omics-Based Clinical Trials Market, By Country, 2020-2028 (USD Million)
73 Saudi Arabia Omics-Based Clinical Trials Market, By Study Design Type, 2020-2028 (USD Million)
74 Saudi Arabia Omics-Based Clinical Trials Market, By Phase Type, 2020-2028 (USD Million)
75 Saudi Arabia Omics-Based Clinical Trials Market, By Indication Type, 2020-2028 (USD Million)
76 UAE Omics-Based Clinical Trials Market, By Study Design Type, 2020-2028 (USD Million)
77 UAE Omics-Based Clinical Trials Market, By Phase Type, 2020-2028 (USD Million)
78 UAE Omics-Based Clinical Trials Market, By Indication Type, 2020-2028 (USD Million)
79 Central & South America Omics-Based Clinical Trials Market, By Study Design Type, 2020-2028 (USD Million)
80 Central & South America Omics-Based Clinical Trials Market, By Phase Type, 2020-2028 (USD Million)
81 Central & South America Omics-Based Clinical Trials Market, By Indication Type, 2020-2028 (USD Million)
82 Central & South America Omics-Based Clinical Trials Market, By Country, 2020-2028 (USD Million)
83 Brazil Omics-Based Clinical Trials Market, By Study Design Type, 2020-2028 (USD Million)
84 Brazil Omics-Based Clinical Trials Market, By Phase Type, 2020-2028 (USD Million)
85 Brazil Omics-Based Clinical Trials Market, By Indication Type, 2020-2028 (USD Million)
86 Pfizer Inc.: Products & Services Offering
87 Covance Inc.: Products & Services Offering
88 Parexel International Corporation: Products & Services Offering
89 Charles River Laboratory: Products & Services Offering
90 Icon Plc: Products & Services Offering
91 ELI LILLY AND COMPANY: Products & Services Offering
92 Rebus Bio : Products & Services Offering
93 Novo Nordisk: Products & Services Offering
94 Other Companies: Products & Services Offering
LIST OF FIGURES
1 Global Omics-Based Clinical Trials Market Overview
2 Global Omics-Based Clinical Trials Market Value From 2020-2028 (USD Mllion)
3 Global Omics-Based Clinical Trials Market Share, By Study Design Type (2022)
4 Global Omics-Based Clinical Trials Market Share, By Phase Type (2022)
5 Global Omics-Based Clinical Trials Market Share, By Indication Type (2022)
6 Global Omics-Based Clinical Trials Market, By Region (Asia Pacific Market)
7 Technological Trends In Global Omics-Based Clinical Trials Market
8 Four Quadrant Competitor Positioning Matrix
9 Impact Of Macro & Micro Indicators On The Market
10 Impact Of Key Drivers On The Global Omics-Based Clinical Trials Market
11 Impact Of Challenges On The Global Omics-Based Clinical Trials Market
12 Porter’s Five Forces Analysis
13 Global Omics-Based Clinical Trials Market: By Study Design Type Scope Key Takeaways
14 Global Omics-Based Clinical Trials Market, By Study Design Type Segment: Revenue Growth Analysis
15 Interventional Studies Market, By Region, 2020-2028 (USD Mllion)
16 Observational Studies Market, By Region, 2020-2028 (USD Mllion)
17 Expanded Access Studies Market, By Region, 2020-2028 (USD Mllion)
18 Global Omics-Based Clinical Trials Market: By Phase Type Scope Key Takeaways
19 Global Omics-Based Clinical Trials Market, By Phase Type Segment: Revenue Growth Analysis
20 Phase I Market, By Region, 2020-2028 (USD Mllion)
21 Phase II Market, By Region, 2020-2028 (USD Mllion)
22 Phase III Market, By Region, 2020-2028 (USD Mllion)
23 Phase IV Market, By Region, 2020-2028 (USD Mllion)
24 Global Omics-Based Clinical Trials Market: By Indication Type Scope Key Takeaways
25 Global Omics-Based Clinical Trials Market, By Indication Type Segment: Revenue Growth Analysis
26 Oncology Market, By Region, 2020-2028 (USD Mllion)
27 Cardiology Market, By Region, 2020-2028 (USD Mllion)
28 Respiratory Diseases Market, By Region, 2020-2028 (USD Mllion)
29 CNS Diseases Market, By Region, 2020-2028 (USD Mllion)
30 Genetic Diseases Market, By Region, 2020-2028 (USD Mllion)
31 Skin Diseases Market, By Region, 2020-2028 (USD Mllion)
32 Other Indications Market, By Region, 2020-2028 (USD Mllion)
33 Regional Segment: Revenue Growth Analysis
34 Global Omics-Based Clinical Trials Market: Regional Analysis
35 North America Omics-Based Clinical Trials Market Overview
36 North America Omics-Based Clinical Trials Market, By Study Design Type
37 North America Omics-Based Clinical Trials Market, By Phase Type
38 North America Omics-Based Clinical Trials Market, By Indication Type
39 North America Omics-Based Clinical Trials Market, By Country
40 U.S. Omics-Based Clinical Trials Market, By Study Design Type
41 U.S. Omics-Based Clinical Trials Market, By Phase Type
42 U.S. Omics-Based Clinical Trials Market, By Indication Type
43 Canada Omics-Based Clinical Trials Market, By Study Design Type
44 Canada Omics-Based Clinical Trials Market, By Phase Type
45 Canada Omics-Based Clinical Trials Market, By Indication Type
46 Mexico Omics-Based Clinical Trials Market, By Study Design Type
47 Mexico Omics-Based Clinical Trials Market, By Phase Type
48 Mexico Omics-Based Clinical Trials Market, By Indication Type
49 Four Quadrant Positioning Matrix
50 Company Market Share Analysis
51 Pfizer Inc.: Company Snapshot
52 Pfizer Inc.: SWOT Analysis
53 Pfizer Inc.: Geographic Presence
54 Covance Inc.: Company Snapshot
55 Covance Inc.: SWOT Analysis
56 Covance Inc.: Geographic Presence
57 Parexel International Corporation: Company Snapshot
58 Parexel International Corporation: SWOT Analysis
59 Parexel International Corporation: Geographic Presence
60 Charles River Laboratory: Company Snapshot
61 Charles River Laboratory: Swot Analysis
62 Charles River Laboratory: Geographic Presence
63 Icon Plc: Company Snapshot
64 Icon Plc: SWOT Analysis
65 Icon Plc: Geographic Presence
66 Eli Lilly and Company: Company Snapshot
67 Eli Lilly and Company: SWOT Analysis
68 Eli Lilly and Company: Geographic Presence
69 Rebus Bio : Company Snapshot
70 Rebus Bio : SWOT Analysis
71 Rebus Bio : Geographic Presence
72 Novo Nordisk: Company Snapshot
73 Novo Nordisk: SWOT Analysis
74 Novo Nordisk: Geographic Presence
75 Other Companies: Company Snapshot
76 Other Companies: SWOT Analysis
77 Other Companies: Geographic Presence
The Global Omics-Based Clinical Trials Market has been studied from the year 2019 till 2028. However, the CAGR provided in the report is from the year 2023 to 2028. The research methodology involved three stages: Desk research, Primary research, and Analysis & Output from the entire research process.

The desk research involved a robust background study which meant referring to paid and unpaid databases to understand the market dynamics; mapping contracts from press releases; identifying the key players in the market, studying their product portfolio, competition level, annual reports/SEC filings & investor presentations; and learning the demand and supply-side analysis for the Omics-Based Clinical Trials Market.

The primary research activity included telephonic conversations with more than 50 tier 1 industry consultants, distributors, and end-use product manufacturers.

Finally, based on the above thorough research process, an in-depth analysis was carried out considering the following aspects: market attractiveness, current & future market trends, market share analysis, SWOT analysis of the company and customer analytics.

Frequently Asked Questions
Tailor made solutions just for you
80% of our clients seek made-to-order reports. How do you want us to tailor yours?
OUR CLIENTS